Electroplating involves depositing a thin layer of metal onto a substrate to enhance surface properties such as corrosion resistance and appearance, while electroforming builds up a thick metal part by electrodeposition on a mold that is later removed. Electroforming allows for the creation of intricate, self-supporting metal structures with precise dimensional control, unlike electroplating, which is primarily surface-focused. Understanding the differences in thickness, structural integrity, and application helps determine the appropriate process for manufacturing and decorative uses in metalworking.
Table of Comparison
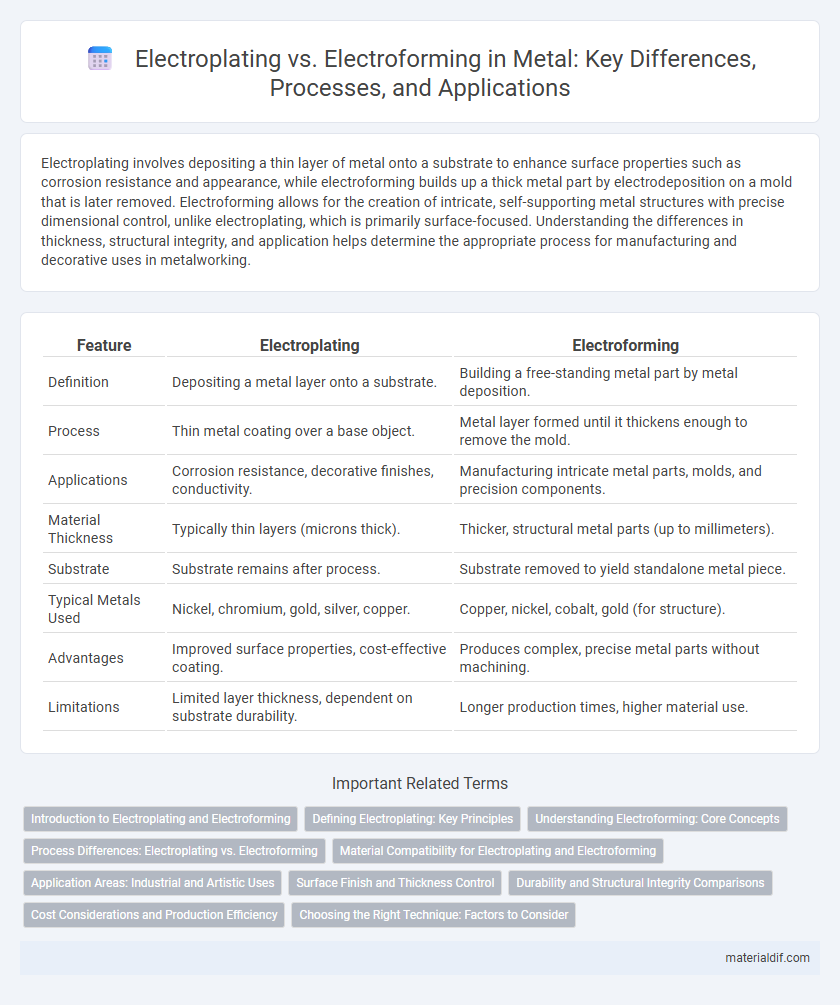
| Feature | Electroplating | Electroforming |
|---|---|---|
| Definition | Depositing a metal layer onto a substrate. | Building a free-standing metal part by metal deposition. |
| Process | Thin metal coating over a base object. | Metal layer formed until it thickens enough to remove the mold. |
| Applications | Corrosion resistance, decorative finishes, conductivity. | Manufacturing intricate metal parts, molds, and precision components. |
| Material Thickness | Typically thin layers (microns thick). | Thicker, structural metal parts (up to millimeters). |
| Substrate | Substrate remains after process. | Substrate removed to yield standalone metal piece. |
| Typical Metals Used | Nickel, chromium, gold, silver, copper. | Copper, nickel, cobalt, gold (for structure). |
| Advantages | Improved surface properties, cost-effective coating. | Produces complex, precise metal parts without machining. |
| Limitations | Limited layer thickness, dependent on substrate durability. | Longer production times, higher material use. |
Introduction to Electroplating and Electroforming
Electroplating involves depositing a thin layer of metal onto a substrate using an electric current to enhance corrosion resistance, appearance, or electrical conductivity. Electroforming is similar but focuses on building thick, self-supporting metal parts by depositing metal onto a mandrel that is later removed. Both processes utilize electrolytic cells but differ primarily in coating thickness and final product structure.
Defining Electroplating: Key Principles
Electroplating involves depositing a thin layer of metal onto a substrate by passing an electric current through an electrolyte solution containing metal ions, enhancing surface properties such as corrosion resistance and aesthetic appeal. This process relies on the reduction of metal cations at the cathode, allowing precise control over coating thickness and uniformity. Key parameters include voltage, current density, and electrolyte composition, which directly impact the quality and adhesion of the plated metal layer.
Understanding Electroforming: Core Concepts
Electroforming is a precise metal fabrication process that deposits metal onto a conductive mold to create intricate, seamless components with high dimensional accuracy. Unlike electroplating, which simply coats a surface with a thin layer of metal, electroforming builds up a thick, self-supporting metal structure by controlled electrodeposition using metals such as copper, nickel, or gold. This technique enables the production of complex geometries and lightweight, durable metal parts critical in aerospace, electronics, and medical device manufacturing.
Process Differences: Electroplating vs. Electroforming
Electroplating involves depositing a thin metal layer onto a substrate through an electrolytic process, enhancing surface properties without altering the object's shape. Electroforming builds metal layers to create a standalone, often intricate, metal part by electrodepositing metal onto a removable mandrel or pattern. The primary distinction lies in electroplating modifying existing surfaces, whereas electroforming fabricates entire metal components from scratch.
Material Compatibility for Electroplating and Electroforming
Electroplating is compatible with a wide range of base metals including steel, copper, brass, and aluminum, as it deposits a thin metallic layer without altering the substrate's shape. Electroforming requires substrates that can withstand longer build times and high current densities, commonly using conductive mandrels made of metal, graphite, or plated plastic to precisely form complex, self-supporting metal structures. Material compatibility in electroforming demands substrate resilience to ensure uniform metal deposition, while electroplating emphasizes adhesion and corrosion resistance on diverse conductive surfaces.
Application Areas: Industrial and Artistic Uses
Electroplating is widely used in automotive, electronics, and aerospace industries for corrosion resistance and aesthetic metal finishes, while electroforming is preferred in precision applications like microfabrication and custom jewelry due to its ability to create complex, hollow metal parts. Artistic uses of electroplating include restoring and enhancing sculptures and decorative objects with thin metal coatings, whereas electroforming enables artists to produce intricate, lightweight metal sculptures and unique textures. Both techniques support industrial manufacturing and artistic creativity but differ in application scale and complexity.
Surface Finish and Thickness Control
Electroplating provides a thin, uniform layer of metal that improves surface finish with precise control over thickness, typically ranging from a few microns to several micrometers. Electroforming allows for the creation of thicker, self-supporting metal parts with superior dimensional accuracy and smooth surface texture, often used in producing intricate or high-precision components. Surface finish quality in electroforming is enhanced by the gradual buildup of metal, enabling better control of microstructure compared to the usually thinner electroplated coatings.
Durability and Structural Integrity Comparisons
Electroplating involves depositing a thin metal layer onto a substrate, enhancing surface durability but providing limited structural reinforcement. Electroforming creates a thicker, self-supporting metal layer, resulting in superior structural integrity and increased resistance to wear and mechanical stress. The choice between the two depends on application requirements, with electroforming preferred for robust, load-bearing components.
Cost Considerations and Production Efficiency
Electroplating offers a cost-effective solution for enhancing metal surfaces by depositing a thin layer of metal, resulting in lower material costs and faster cycle times compared to electroforming. Electroforming involves building up thick metal layers to create intricate, standalone parts, which can increase production time and expenses due to higher material usage and specialized equipment requirements. Manufacturers must weigh the reduced cost and speed benefits of electroplating against the precision and structural advantages of electroforming for efficient metal fabrication.
Choosing the Right Technique: Factors to Consider
Electroplating is ideal for enhancing surface properties by depositing a thin metal layer on a substrate, improving corrosion resistance and aesthetics, while electroforming builds thicker, self-supporting metal parts suitable for complex geometries and precision components. Key factors in choosing the right technique include the desired metal thickness, structural integrity requirements, and application purpose, such as decorative versus functional use. Cost considerations, production volume, and material compatibility also influence the decision between electroplating and electroforming processes.
Electroplating vs Electroforming Infographic
 materialdif.com
materialdif.com