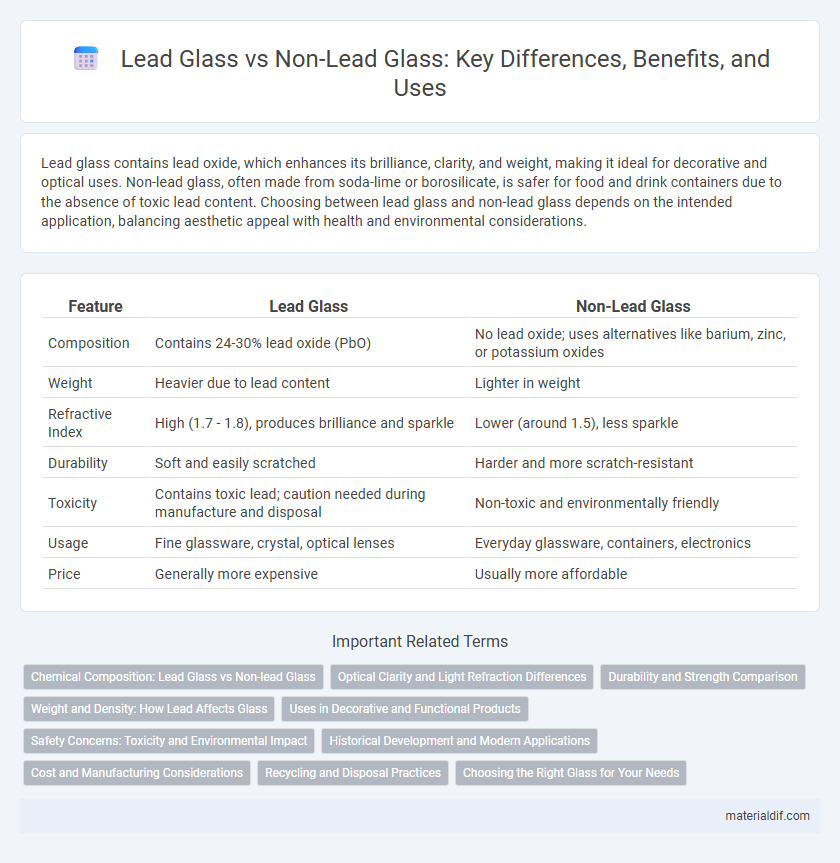
Lead glass contains lead oxide, which enhances its brilliance, clarity, and weight, making it ideal for decorative and optical uses. Non-lead glass, often made from soda-lime or borosilicate, is safer for food and drink containers due to the absence of toxic lead content. Choosing between lead glass and non-lead glass depends on the intended application, balancing aesthetic appeal with health and environmental considerations.
Table of Comparison
| Feature | Lead Glass | Non-Lead Glass |
|---|---|---|
| Composition | Contains 24-30% lead oxide (PbO) | No lead oxide; uses alternatives like barium, zinc, or potassium oxides |
| Weight | Heavier due to lead content | Lighter in weight |
| Refractive Index | High (1.7 - 1.8), produces brilliance and sparkle | Lower (around 1.5), less sparkle |
| Durability | Soft and easily scratched | Harder and more scratch-resistant |
| Toxicity | Contains toxic lead; caution needed during manufacture and disposal | Non-toxic and environmentally friendly |
| Usage | Fine glassware, crystal, optical lenses | Everyday glassware, containers, electronics |
| Price | Generally more expensive | Usually more affordable |
Chemical Composition: Lead Glass vs Non-lead Glass
Lead glass contains high concentrations of lead oxide, typically ranging from 18% to 40%, which enhances its refractive index and brilliance. Non-lead glass primarily uses alkali oxides such as sodium oxide and calcium oxide, resulting in lower density and altered optical properties. The chemical composition differences directly impact durability, clarity, and weight in glass products.
Optical Clarity and Light Refraction Differences
Lead glass exhibits superior optical clarity due to its high refractive index, typically around 1.7 to 1.8, which enhances brightness and sparkle by bending light more effectively than non-lead glass with refractive indices near 1.5. This increased light refraction in lead glass creates a distinctive brilliance and sharper visual effects, making it preferred for decorative and luxury applications. Non-lead glass, while safer and more environmentally friendly, lacks this high refractive quality, resulting in less intense light dispersion and a clearer but less vibrant appearance.
Durability and Strength Comparison
Lead glass exhibits higher density and enhanced refractive properties, making it more resistant to mechanical stress and less prone to breakage compared to non-lead glass. Non-lead glass, typically composed of silica and alkali oxides, offers lower durability and strength but provides better environmental safety due to the absence of toxic lead content. The increased durability of lead glass makes it preferable for decorative and optical applications, while non-lead glass is often favored in everyday use where safety and recyclability are priorities.
Weight and Density: How Lead Affects Glass
Lead glass, also known as lead crystal, contains a significant percentage of lead oxide, typically between 24% and 32%, which drastically increases its density and weight compared to non-lead glass. Non-lead glass, often made from silica, soda, and lime, has a lower density, generally around 2.4 g/cm3, whereas lead glass can reach densities of 3.1 to 3.4 g/cm3. The added lead content enhances the refractive index and brilliance of the glass but also makes it heavier and denser, affecting its handling and use in various applications.
Uses in Decorative and Functional Products
Lead glass, prized for its high refractive index and brilliance, is extensively used in decorative products such as fine crystalware, chandeliers, and luxury vases, providing enhanced clarity and sparkle. Non-lead glass, favored for its safety and environmental benefits, is commonly utilized in functional items like food containers, laboratory glassware, and optical lenses, where durability and non-toxicity are critical. Both types serve distinct purposes, with lead glass excelling in aesthetic appeal and non-lead glass optimized for everyday practical applications.
Safety Concerns: Toxicity and Environmental Impact
Lead glass contains lead oxide, which enhances clarity and weight but poses significant toxicity risks if broken or improperly disposed of, potentially contaminating soil and water sources. Non-lead glass, typically made from silica and other non-toxic additives, offers a safer alternative with less environmental impact due to its inert composition and recyclability. Regulatory agencies often restrict the use of lead glass in food and drink containers due to the leaching of lead, underscoring the importance of safer glass alternatives for consumer products.
Historical Development and Modern Applications
Lead glass, developed in the 17th century by George Ravenscroft, revolutionized glassmaking with its high refractive index and brilliance, making it a staple in luxury tableware and optical lenses. Non-lead glass emerged as an environmental and health-conscious alternative, utilizing borosilicate and other compositions to offer durability and thermal resistance for laboratory equipment and consumer products. Modern applications prioritize non-lead glass for eco-friendly packaging and electronic displays, while lead glass maintains a niche for radiation shielding and high-end crystal craftsmanship.
Cost and Manufacturing Considerations
Lead glass typically costs more due to the inclusion of lead oxide, which enhances clarity and weight but raises production expenses and environmental concerns during manufacturing. Non-lead glass, often made with alternatives like barium or zinc, offers lower manufacturing costs and reduced health risks, making it a more economical and eco-friendly choice. The complexity of sourcing lead and the need for specialized disposal methods further increase lead glass production costs compared to non-lead varieties.
Recycling and Disposal Practices
Lead glass requires specialized recycling processes to safely handle its toxic lead content, preventing environmental contamination and health risks. Non-lead glass is more commonly accepted in standard recycling streams due to its non-toxic composition, facilitating more efficient and widespread material recovery. Proper disposal of lead glass involves designated hazardous waste facilities to avoid lead leaching into soil and water, whereas non-lead glass can be disposed of in regular glass recycling bins without special precautions.
Choosing the Right Glass for Your Needs
Lead glass contains lead oxide, enhancing its brilliance, weight, and refractive index, making it ideal for luxury glassware and optical instruments. Non-lead glass, often made with barium or potassium, offers greater environmental safety and durability, suitable for everyday use and food storage. Selecting the right glass depends on balancing aesthetic preference, health considerations, and intended functionality.
Lead glass vs Non-lead glass Infographic
 materialdif.com
materialdif.com