Class 1 nickel, also known as primary nickel, is produced through pyrometallurgical or hydrometallurgical processes and is characterized by high purity levels, making it ideal for demanding applications such as stainless steel manufacturing and batteries. Class 2 nickel consists of nickel pig iron or ferronickel, derived mainly from laterite ores with lower nickel content and impurities, primarily used in alloy production where purity is less critical. Understanding the differences in composition, processing methods, and end-use applications is essential for optimizing material selection and cost-efficiency in nickel-based industries.
Table of Comparison
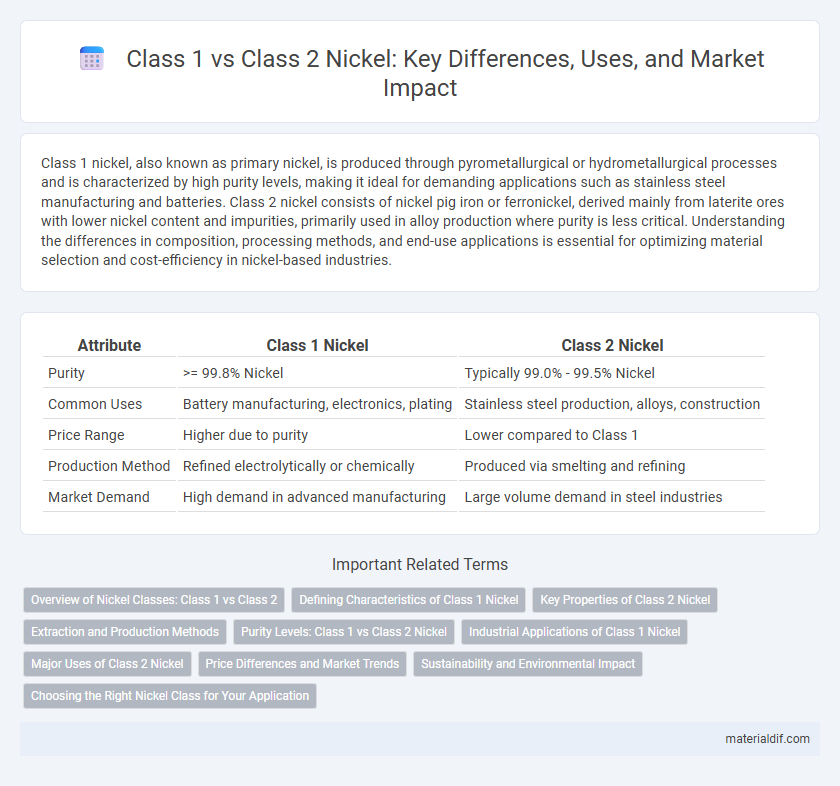
| Attribute | Class 1 Nickel | Class 2 Nickel |
|---|---|---|
| Purity | >= 99.8% Nickel | Typically 99.0% - 99.5% Nickel |
| Common Uses | Battery manufacturing, electronics, plating | Stainless steel production, alloys, construction |
| Price Range | Higher due to purity | Lower compared to Class 1 |
| Production Method | Refined electrolytically or chemically | Produced via smelting and refining |
| Market Demand | High demand in advanced manufacturing | Large volume demand in steel industries |
Overview of Nickel Classes: Class 1 vs Class 2
Class 1 nickel is high-purity nickel containing at least 99.8% nickel, primarily used in stainless steel production, batteries, and plating applications due to its superior corrosion resistance and electrical conductivity. Class 2 nickel, with lower purity levels and containing significant amounts of iron and other impurities, is typically employed in alloy production and other industrial uses where high purity is not critical. The distinction between Class 1 and Class 2 nickel influences their market demand, pricing structures, and suitability for specific manufacturing processes.
Defining Characteristics of Class 1 Nickel
Class 1 nickel is characterized by its high purity, typically containing over 99% nickel, making it ideal for applications requiring corrosion resistance and long-term durability such as stainless steel production and specialty alloys. This type of nickel is extracted primarily through the laterite and sulphide ore refining processes, ensuring minimal impurities like iron or cobalt. Class 1 nickel's superior electrochemical properties distinguish it from Class 2 nickel, which mainly comprises nickel pig iron or ferronickel with lower nickel content and is primarily used in lower-grade stainless steel or battery manufacturing.
Key Properties of Class 2 Nickel
Class 2 nickel primarily consists of nickel-containing alloys with lower purity, typically around 99.8% nickel, compared to Class 1 nickel, which is defined by a higher purity of 99.8% or greater. Key properties of Class 2 nickel include enhanced mechanical strength, corrosion resistance, and improved workability due to the presence of alloying elements such as iron, copper, and cobalt. This makes Class 2 nickel ideal for applications requiring durability and resistance to harsh environments, including batteries, stainless steel production, and industrial components.
Extraction and Production Methods
Class 1 nickel is primarily produced through laterite ore processing using high-pressure acid leaching (HPAL) or from sulfide ores via pyrometallurgical smelting and refining, yielding high-purity nickel suitable for batteries and electronics. Class 2 nickel, derived mainly from low-grade laterite ores and mixed sulfide sources, involves hydrometallurgical or aluminothermic reduction methods, resulting in nickel pig iron or ferronickel with lower purity levels. Extraction efficiency, ore grade, and processing complexity significantly differentiate Class 1 from Class 2 nickel in terms of production scalability and end-use application.
Purity Levels: Class 1 vs Class 2 Nickel
Class 1 nickel features a purity level of over 99.8%, making it ideal for high-grade applications such as stainless steel production and specialty alloys. Class 2 nickel contains impurities that reduce its purity below 99%, typically used in lower-grade applications like catalysts and batteries. The distinct purity levels directly influence the performance, market value, and processing methods of nickel in industrial use.
Industrial Applications of Class 1 Nickel
Class 1 nickel, featuring a minimum purity of 99.8%, is predominantly utilized in industrial applications requiring superior corrosion resistance and high mechanical strength, such as stainless steel production, aerospace components, and electronics. Its exceptional ductility and thermal stability make it the preferred choice in chemical processing equipment, batteries for electric vehicles, and hydrogen storage systems. In contrast, Class 2 nickel, with lower purity and higher iron content, is mainly used in less demanding industrial applications where cost efficiency outweighs performance.
Major Uses of Class 2 Nickel
Class 2 nickel primarily serves as an essential alloying element in the production of stainless steel and various industrial metals, where its corrosion resistance and strength enhance durability. It is widely used in manufacturing components for automotive, aerospace, and construction industries due to its cost-effective properties compared to Class 1 nickel. Class 2 nickel also finds applications in plating and battery materials, supporting the growth of electric vehicle technologies and other energy storage solutions.
Price Differences and Market Trends
Class 1 nickel, characterized by its high purity (99.8% and above), commands a premium price due to its essential role in stainless steel production and electric vehicle batteries. Class 2 nickel, with lower purity and often sourced from lateritic ores, generally trades at a discount reflecting higher processing costs and limited industrial applications. Market trends reveal increasing demand for Class 1 nickel driven by the EV sector, causing price differentials to widen as supply struggles to keep pace with clean nickel requirements.
Sustainability and Environmental Impact
Class 1 nickel, characterized by its high purity and suitability for stainless steel and battery production, promotes sustainability through efficient recycling processes and lower carbon emissions during refining. Class 2 nickel, primarily used in alloys and plating, often involves more energy-intensive extraction methods and higher environmental impact due to impurities and less efficient processing. Prioritizing Class 1 nickel supports the transition to greener technologies, enhancing environmental performance in critical applications like electric vehicle batteries.
Choosing the Right Nickel Class for Your Application
Class 1 nickel offers high purity (99.8% or greater) and excellent corrosion resistance, making it ideal for aerospace, electronics, and chemical processing industries where precise performance is critical. Class 2 nickel contains lower purity levels and is commonly mixed with other metals, suitable for applications like stainless steel production and alloy manufacturing where cost efficiency is prioritized. Selecting the right nickel class depends on balancing purity requirements, mechanical properties, and budget constraints to optimize durability and functionality in specific industrial uses.
Class 1 nickel vs Class 2 nickel Infographic
 materialdif.com
materialdif.com