Lead and bismuth are both heavy metals with distinct environmental and health impacts; lead is highly toxic and poses significant health risks, especially to children, due to its neurotoxic effects. Bismuth offers a safer, non-toxic alternative with similar density, making it preferable for applications like fishing weights and ammunition where toxicity is a concern. Choosing bismuth reduces environmental contamination and ensures safer handling, aligning with increasing regulations against lead use.
Table of Comparison
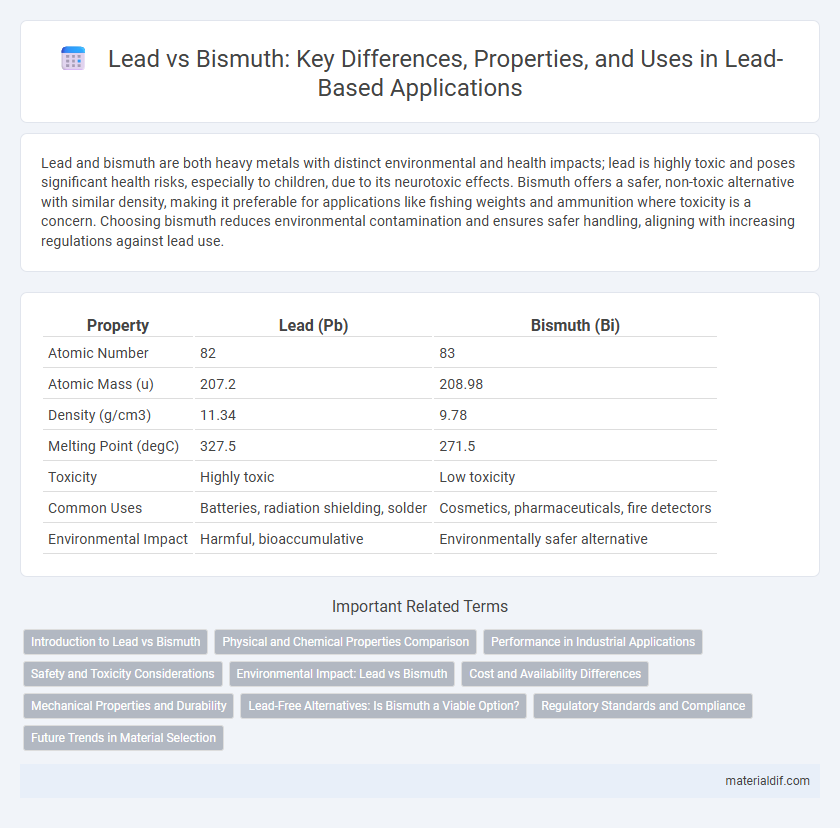
| Property | Lead (Pb) | Bismuth (Bi) |
|---|---|---|
| Atomic Number | 82 | 83 |
| Atomic Mass (u) | 207.2 | 208.98 |
| Density (g/cm3) | 11.34 | 9.78 |
| Melting Point (degC) | 327.5 | 271.5 |
| Toxicity | Highly toxic | Low toxicity |
| Common Uses | Batteries, radiation shielding, solder | Cosmetics, pharmaceuticals, fire detectors |
| Environmental Impact | Harmful, bioaccumulative | Environmentally safer alternative |
Introduction to Lead vs Bismuth
Lead and bismuth are both post-transition metals with similar physical properties, such as high density and softness. Lead is widely used in batteries, radiation shielding, and construction, while bismuth serves as a non-toxic replacement for lead in pharmaceuticals and cosmetics. Despite their chemical similarities, bismuth has a lower toxicity and unique electronic structure, making it valuable in specialized applications.
Physical and Chemical Properties Comparison
Lead and bismuth are dense, post-transition metals with similar atomic numbers, 82 and 83 respectively, but lead has a higher density of 11.34 g/cm3 compared to bismuth's 9.78 g/cm3. Chemically, lead exhibits a +2 and +4 oxidation state with greater reactivity, forming compounds such as lead oxide and lead acetate, while bismuth is less reactive, commonly showing a +3 state and forming stable oxybismuth compounds. Both metals have low melting points relative to other metals, with lead melting at 327.5degC and bismuth at 271.5degC, but bismuth expands upon solidification, a rare physical property not shared by lead.
Performance in Industrial Applications
Lead exhibits superior corrosion resistance and higher density compared to bismuth, enhancing its performance in radiation shielding and battery manufacturing. Bismuth offers lower toxicity and better machinability, making it suitable for specialized applications such as low-melting alloys and pharmaceuticals. In industrial environments demanding durability under harsh conditions, lead remains the preferred choice despite environmental concerns.
Safety and Toxicity Considerations
Lead exposure presents significant health hazards due to its neurotoxicity and potential to cause cognitive impairments, whereas bismuth is considered far less toxic and possesses a better safety profile in medical and industrial applications. Lead accumulates in the body, particularly affecting the nervous system and causing long-term damage, while bismuth compounds exhibit low bioavailability and are often used safely in pharmaceuticals. Strict regulatory limits govern lead exposure to prevent poisoning, whereas bismuth's toxicity concerns are minimal, making it a safer alternative in contexts requiring heavy metal use.
Environmental Impact: Lead vs Bismuth
Lead is highly toxic to the environment, accumulating in soil and water and posing serious risks to wildlife and human health due to its neurotoxic properties. Bismuth offers a more eco-friendly alternative, as it is less toxic and biodegradable, reducing contamination in ecosystems. The environmental impact of lead remains a critical concern in industrial applications, prompting a shift towards bismuth for safer, sustainable practices.
Cost and Availability Differences
Lead is generally more affordable and widely available compared to bismuth, making it a preferred choice in large-scale industrial applications. Bismuth, being rarer and less abundant, typically incurs higher costs and limited supply chains. The price disparity stems from bismuth's scarcity in the earth's crust and more complex extraction processes relative to lead.
Mechanical Properties and Durability
Lead exhibits lower tensile strength and hardness compared to bismuth, making it more malleable but less resistant to mechanical stress. Bismuth offers superior durability with higher corrosion resistance and better dimensional stability under mechanical loads. These properties make bismuth more suitable for applications requiring long-term structural integrity and mechanical reliability.
Lead-Free Alternatives: Is Bismuth a Viable Option?
Bismuth emerges as a promising lead-free alternative due to its non-toxicity and similar malleability, making it suitable for applications where lead's health risks are prohibitive. Its higher melting point and comparable density allow bismuth to replace lead in solders, radiation shielding, and metal alloys without compromising performance. Despite cost considerations, bismuth's environmental benefits position it as a viable option in industries striving to eliminate lead usage.
Regulatory Standards and Compliance
Lead and bismuth differ significantly in regulatory standards due to their varying toxicity levels. Lead is subject to strict regulations under agencies like the EPA and OSHA, which limit its use and mandate rigorous handling and disposal procedures to prevent environmental contamination and human health risks. Bismuth, being less toxic, faces fewer restrictions, allowing broader applications in pharmaceuticals and cosmetics while still complying with general chemical safety standards.
Future Trends in Material Selection
Future trends in material selection indicate a shift from lead to bismuth due to bismuth's non-toxic and environmentally friendly properties. Bismuth's increasing application in electronics, medical devices, and alloys highlights its potential to replace lead in various industries. Advancements in sustainable materials science emphasize bismuth's role in meeting regulatory demands and enhancing performance while minimizing health risks.
Lead vs Bismuth Infographic
 materialdif.com
materialdif.com