Gypsum contains water molecules within its crystal structure, making it softer and easier to work with compared to anhydrite, which lacks water and is denser and harder. Anhydrite is less soluble in water than gypsum, influencing its applications in construction and agriculture. Both minerals share a chemical similarity but differ significantly in physical properties and behavior when exposed to moisture.
Table of Comparison
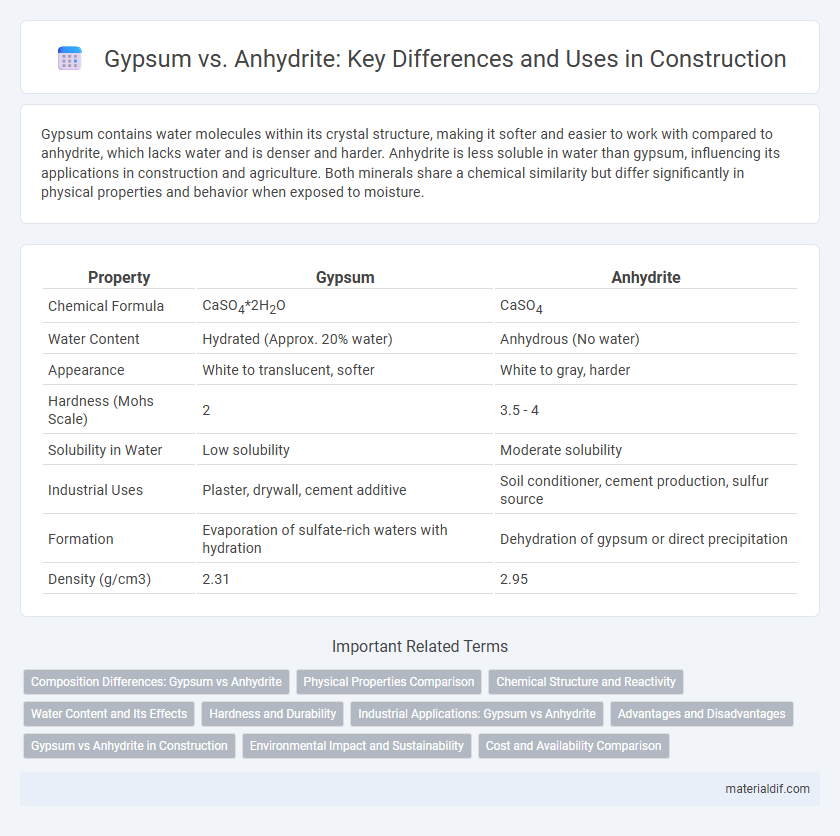
| Property | Gypsum | Anhydrite |
|---|---|---|
| Chemical Formula | CaSO4*2H2O | CaSO4 |
| Water Content | Hydrated (Approx. 20% water) | Anhydrous (No water) |
| Appearance | White to translucent, softer | White to gray, harder |
| Hardness (Mohs Scale) | 2 | 3.5 - 4 |
| Solubility in Water | Low solubility | Moderate solubility |
| Industrial Uses | Plaster, drywall, cement additive | Soil conditioner, cement production, sulfur source |
| Formation | Evaporation of sulfate-rich waters with hydration | Dehydration of gypsum or direct precipitation |
| Density (g/cm3) | 2.31 | 2.95 |
Composition Differences: Gypsum vs Anhydrite
Gypsum is primarily composed of calcium sulfate dihydrate (CaSO4*2H2O), while anhydrite consists of calcium sulfate without water (CaSO4). This fundamental difference in hydration level impacts their physical properties, with gypsum being softer and more hydrated compared to the dry, denser anhydrite. The presence or absence of water molecules in their crystal structure defines their distinct industrial applications and behavior in natural environments.
Physical Properties Comparison
Gypsum is a soft sulfate mineral with a Mohs hardness of 2 and a specific gravity around 2.3, while anhydrite is harder with a Mohs hardness of 3 to 3.5 and a specific gravity ranging from 2.9 to 3.0. Gypsum typically exhibits perfect cleavage in one direction and a fibrous to granular crystal habit, contrasting with anhydrite's more massive to granular habit and distinct cleavage in three directions. Both minerals are transparent to translucent, but gypsum often appears pearly or silky due to its hydration, whereas anhydrite lacks water and has a duller luster.
Chemical Structure and Reactivity
Gypsum (CaSO4*2H2O) contains two water molecules in its crystal structure, while anhydrite (CaSO4) is its anhydrous counterpart, lacking water. The presence of water in gypsum increases its reactivity and solubility, making it more adaptable for hydration reactions in construction applications. Anhydrite, with its denser and more stable chemical structure, exhibits lower reactivity and slower setting times in cementitious mixtures.
Water Content and Its Effects
Gypsum contains about 20-21% water by weight in the form of crystalline water, which significantly influences its hardness and solubility, making it softer and more reactive in moist environments. Anhydrite, lacking this water, is much harder and less soluble, displaying increased stability in dry conditions but slower rehydration rates. The water content in gypsum also affects its industrial applications, especially in construction and agriculture, where moisture retention and reactivity are critical factors.
Hardness and Durability
Gypsum exhibits a Mohs hardness of 2, making it softer and more prone to scratching compared to anhydrite, which has a hardness of approximately 3 to 3.5. Durability-wise, gypsum is less resistant to weathering and chemical breakdown, while anhydrite's denser crystal structure provides better durability in construction and industrial applications. These differences influence their suitability, with gypsum favored for ease of shaping and anhydrite preferred where higher strength and longevity are critical.
Industrial Applications: Gypsum vs Anhydrite
Gypsum and anhydrite serve distinct roles in industrial applications, with gypsum primarily used in construction for drywall, plaster, and cement production due to its ease of hydration and setting properties. Anhydrite, being denser and less soluble, is favored in applications requiring lower water content and slower setting times, such as soil stabilization and as a drying agent in the chemical industry. The choice between gypsum and anhydrite depends on hydration characteristics, solubility, and mechanical properties tailored to specific industrial needs.
Advantages and Disadvantages
Gypsum, a hydrated calcium sulfate mineral, offers advantages such as ease of use, excellent fire resistance, and superior moisture regulation compared to anhydrite, which lacks water molecules and thus has lower workability and higher dust generation. Anhydrite's denser structure provides greater compressive strength and faster setting times, but it is less adaptable for indoor environments due to its sensitivity to moisture. Gypsum's balance of durability and moisture tolerance makes it preferable for drywall and plaster applications, whereas anhydrite is often favored in industrial and flooring contexts where high strength and rapid curing are critical.
Gypsum vs Anhydrite in Construction
Gypsum and anhydrite differ primarily in their water content, with gypsum containing crystalline water and anhydrite lacking it, affecting their behavior in construction applications. Gypsum's higher water content allows it to be more workable and readily used for plaster, drywall, and cement additives, providing enhanced fire resistance and sound insulation. Anhydrite, being denser and less porous, is often favored for floor screeds due to its dimensional stability and rapid setting time, making the choice dependent on specific structural and environmental requirements.
Environmental Impact and Sustainability
Gypsum is more environmentally friendly than anhydrite due to its lower carbon footprint and easier recyclability in construction applications. The natural hydration process of gypsum allows for reduced energy consumption during production compared to anhydrite, which requires additional calcination. Sustainable construction practices favor gypsum because it supports better indoor air quality by regulating humidity, whereas anhydrite's higher processing emissions contribute more significantly to environmental pollution.
Cost and Availability Comparison
Gypsum is generally more cost-effective than anhydrite due to its widespread natural deposits and easier mining process. Anhydrite, being less abundant and requiring more energy-intensive extraction, tends to have higher prices and limited availability. Construction and agricultural industries often prefer gypsum for its affordability and consistent supply.
Gypsum vs Anhydrite Infographic
 materialdif.com
materialdif.com