Gypsum soil amendment improves soil structure by breaking up compacted clay and enhancing water infiltration without significantly altering soil pH, making it ideal for calcium-deficient, saline, or sodic soils. Lime soil amendment primarily raises soil pH by neutralizing acidity, which benefits nutrient availability but may not improve soil physical properties. Choosing gypsum or lime depends on whether the goal is to enhance soil structure and calcium levels or to correct soil acidity for optimal plant growth.
Table of Comparison
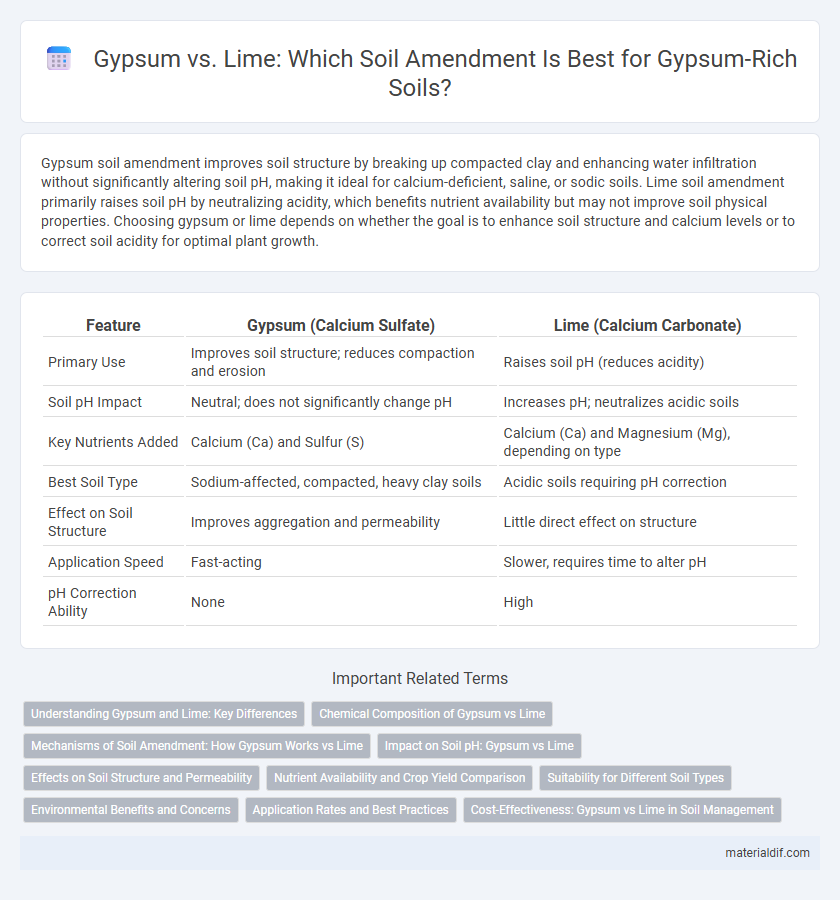
| Feature | Gypsum (Calcium Sulfate) | Lime (Calcium Carbonate) |
|---|---|---|
| Primary Use | Improves soil structure; reduces compaction and erosion | Raises soil pH (reduces acidity) |
| Soil pH Impact | Neutral; does not significantly change pH | Increases pH; neutralizes acidic soils |
| Key Nutrients Added | Calcium (Ca) and Sulfur (S) | Calcium (Ca) and Magnesium (Mg), depending on type |
| Best Soil Type | Sodium-affected, compacted, heavy clay soils | Acidic soils requiring pH correction |
| Effect on Soil Structure | Improves aggregation and permeability | Little direct effect on structure |
| Application Speed | Fast-acting | Slower, requires time to alter pH |
| pH Correction Ability | None | High |
Understanding Gypsum and Lime: Key Differences
Gypsum improves soil structure by adding calcium and sulfur without altering soil pH, making it ideal for sodium-affected or saline soils. Lime raises soil pH by neutralizing acidity, supplying calcium or magnesium, which enhances nutrient availability in acidic soils. Understanding these key differences helps tailor soil amendments to specific crop and soil requirements for optimal growth.
Chemical Composition of Gypsum vs Lime
Gypsum (CaSO4*2H2O) primarily contributes calcium and sulfur without significantly altering soil pH, while lime (CaCO3) supplies calcium and magnesium and increases soil pH by neutralizing acidity. Gypsum's calcium is in the form of calcium sulfate, which enhances soil structure and nutrient availability without causing alkalinity, unlike lime's calcium carbonate that reacts with soil acids to raise pH levels. The sulfur in gypsum also serves as a vital plant nutrient, absent in traditional lime amendments, making gypsum beneficial for improving soil chemistry without altering acidity.
Mechanisms of Soil Amendment: How Gypsum Works vs Lime
Gypsum improves soil structure by supplying calcium and sulfur, which flocculate clay particles and enhance water infiltration without significantly altering soil pH. Lime primarily increases soil pH by neutralizing acidity through calcium carbonate, which helps release essential nutrients bound in acidic soils. While lime targets pH correction and nutrient availability, gypsum focuses on improving soil physical properties and calcium levels without raising alkalinity.
Impact on Soil pH: Gypsum vs Lime
Gypsum is calcium sulfate and does not significantly alter soil pH, making it ideal for improving soil structure without increasing alkalinity. Lime, primarily calcium carbonate, raises soil pH by neutralizing acidity, which enhances nutrient availability in acidic soils. Selecting gypsum over lime benefits soils requiring calcium without the risk of pH imbalance.
Effects on Soil Structure and Permeability
Gypsum improves soil structure by promoting calcium ion exchange, which helps flocculate clay particles and enhances soil aggregation, leading to better aeration and water infiltration. Lime primarily adjusts soil pH by neutralizing acidity but has limited direct impact on soil permeability or aggregation. Gypsum is especially effective in sodic or saline soils where improved permeability and reduced crusting are critical for root growth and water movement.
Nutrient Availability and Crop Yield Comparison
Gypsum soil amendment improves nutrient availability by supplying calcium and sulfur without altering soil pH, enhancing nutrient uptake in crops grown in alkaline and sodic soils. Lime soil amendment primarily raises soil pH, making phosphorus and micronutrients more available but may cause calcium to precipitate in high pH conditions. Studies show gypsum can increase crop yield by improving soil structure and sulfur nutrition, while lime boosts yield mainly through pH correction and phosphorus availability in acid soils.
Suitability for Different Soil Types
Gypsum is highly suitable for sodic and clay soils, improving soil structure by breaking up compacted layers without altering pH significantly. Lime is more effective for acidic soils, raising pH levels to enhance nutrient availability and microbial activity. Choosing between gypsum and lime depends on specific soil conditions such as pH imbalance and sodicity.
Environmental Benefits and Concerns
Gypsum improves soil structure by supplying calcium and sulfur without significantly altering pH, making it suitable for sodic and saline soils while reducing aluminum toxicity and improving water infiltration. Lime raises soil pH by neutralizing acidity, which enhances nutrient availability but can increase carbon dioxide emissions due to chemical reactions in the soil. Gypsum application minimizes the risk of nutrient leaching and supports sustainable soil management, whereas lime's environmental concerns include potential overliming and negative impacts on soil microbial communities.
Application Rates and Best Practices
Gypsum soil amendment typically requires application rates of 20 to 50 pounds per 1,000 square feet to improve soil structure and alleviate sodium toxicity without significantly altering soil pH. Lime application rates are usually higher, ranging from 40 to 100 pounds per 1,000 square feet, as lime primarily raises soil pH by neutralizing acidity and supplying calcium and magnesium. Best practices recommend applying gypsum in soils with high sodium content or poor drainage, while lime is optimal for acidic soils requiring pH adjustment and nutrient availability enhancement.
Cost-Effectiveness: Gypsum vs Lime in Soil Management
Gypsum soil amendment offers a cost-effective solution for improving soil structure and alleviating sodicity, often requiring lower application rates compared to lime. Lime is generally more affordable per ton but may need higher quantities to adjust soil pH and address calcium deficiencies, increasing overall expense. For soils with high sodium content, gypsum provides targeted benefits with a better cost-to-benefit ratio, while lime remains preferable for widespread pH correction.
Gypsum soil amendment vs Lime soil amendment Infographic
 materialdif.com
materialdif.com