Platinum catalysts offer superior durability and higher resistance to poisoning compared to nickel catalysts, making them ideal for applications requiring long-term stability and efficiency. They provide enhanced catalytic activity and selectivity, resulting in better performance in processes such as hydrogenation and fuel cell reactions. While nickel catalysts are more cost-effective, platinum catalysts deliver significantly improved reaction rates and overall process reliability.
Table of Comparison
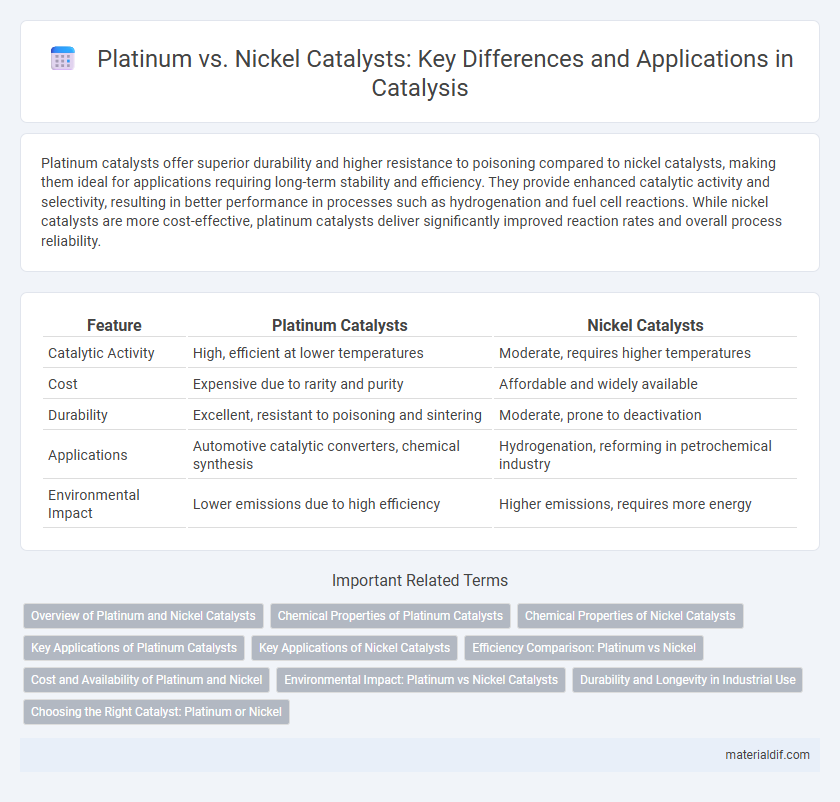
| Feature | Platinum Catalysts | Nickel Catalysts |
|---|---|---|
| Catalytic Activity | High, efficient at lower temperatures | Moderate, requires higher temperatures |
| Cost | Expensive due to rarity and purity | Affordable and widely available |
| Durability | Excellent, resistant to poisoning and sintering | Moderate, prone to deactivation |
| Applications | Automotive catalytic converters, chemical synthesis | Hydrogenation, reforming in petrochemical industry |
| Environmental Impact | Lower emissions due to high efficiency | Higher emissions, requires more energy |
Overview of Platinum and Nickel Catalysts
Platinum catalysts exhibit superior catalytic activity and resistance to poisoning compared to nickel catalysts, making them especially effective in hydrogenation and reforming processes. Nickel catalysts offer cost advantages and are widely used in hydrocracking and methanation but typically require higher temperatures and are more prone to deactivation. The choice between platinum and nickel catalysts depends on reaction conditions, desired selectivity, and economic factors within industrial chemical applications.
Chemical Properties of Platinum Catalysts
Platinum catalysts exhibit superior chemical stability and resistance to poisoning compared to nickel catalysts, enabling enhanced durability in harsh reaction environments. Their unique electronic structure facilitates efficient hydrogen adsorption and activation, resulting in higher catalytic activity for hydrogenation and reforming processes. The inherent resistance of platinum to oxidation and sulfur compounds further ensures prolonged catalyst life and consistent performance.
Chemical Properties of Nickel Catalysts
Nickel catalysts exhibit strong resistance to sulfur poisoning and high thermal stability, making them ideal for processes like hydrogenation and methane reforming. Their surface chemistry involves variable oxidation states, primarily Ni(0) and Ni(2+), facilitating efficient adsorption and activation of hydrogen molecules. Compared to platinum, nickel catalysts have a higher propensity for carbon deposition, which can lead to catalyst deactivation under prolonged reaction conditions.
Key Applications of Platinum Catalysts
Platinum catalysts are extensively used in catalytic converters for automotive exhaust systems, fuel cells, and chemical synthesis processes due to their superior resistance to poisoning and excellent catalytic activity. Unlike nickel catalysts, which are primarily employed in hydrogenation reactions and methanation, platinum catalysts excel in oxidation reactions and reforming processes critical for refining petroleum and producing clean energy. Their stability at high temperatures and ability to facilitate complex reactions make platinum catalysts indispensable in environmental control and hydrogen production technologies.
Key Applications of Nickel Catalysts
Nickel catalysts are extensively used in hydrogenation processes such as the production of margarine and biodiesel, where their cost-effectiveness and high activity are crucial. They play a vital role in reforming hydrocarbons to produce synthetic fuels and chemicals, particularly in the petrochemical industry. Unlike platinum catalysts, which excel in oxidation and fuel cell applications, nickel catalysts dominate in large-scale hydrogenation and reforming due to their lower price and robustness.
Efficiency Comparison: Platinum vs Nickel
Platinum catalysts exhibit significantly higher efficiency than nickel catalysts due to their superior catalytic activity and stability in various chemical reactions, including hydrogenation and oxidation processes. Platinum's ability to operate effectively at lower temperatures reduces energy consumption and enhances reaction rates, making it more suitable for industrial applications requiring precision and durability. Nickel catalysts, while more cost-effective, typically show lower turnover frequencies and shorter lifespans, limiting their efficiency in long-term, high-performance catalytic systems.
Cost and Availability of Platinum and Nickel
Platinum catalysts generally command higher costs due to platinum's rarity and complex extraction process, making it a premium choice for catalytic applications. Nickel catalysts offer a more cost-effective alternative, benefiting from nickel's greater abundance and lower market price, which enhances their accessibility for industrial use. Despite platinum's superior catalytic efficiency, nickel's affordability and wide availability drive its preference in cost-sensitive applications.
Environmental Impact: Platinum vs Nickel Catalysts
Platinum catalysts exhibit higher catalytic efficiency and selectivity, leading to reduced emissions and lower fuel consumption in industrial processes compared to nickel catalysts. Platinum's superior resistance to poisoning and longer lifespan decrease the frequency of catalyst replacement, minimizing environmental waste and resource extraction. Nickel catalysts, while cheaper, often produce higher levels of greenhouse gases and require more frequent disposal, resulting in a greater overall environmental footprint.
Durability and Longevity in Industrial Use
Platinum catalysts exhibit superior durability and longevity compared to nickel catalysts in industrial applications due to their higher resistance to poisoning and thermal degradation. The enhanced stability of platinum enables prolonged catalytic activity, reducing downtime and replacement frequency in processes like hydrogenation and reforming. Nickel catalysts typically suffer from faster deactivation caused by coking and sulfur poisoning, limiting their lifespan under harsh operating conditions.
Choosing the Right Catalyst: Platinum or Nickel
Platinum catalysts offer superior activity and resistance to poisoning, making them ideal for high-performance applications such as fuel cells and automotive catalytic converters. Nickel catalysts provide a cost-effective alternative with good activity for hydrogenation and reforming processes, suitable for large-scale industrial use where budget constraints exist. Selecting the right catalyst depends on balancing performance requirements, operating conditions, and economic factors to optimize efficiency and longevity.
Platinum Catalysts vs Nickel Catalysts Infographic
 materialdif.com
materialdif.com