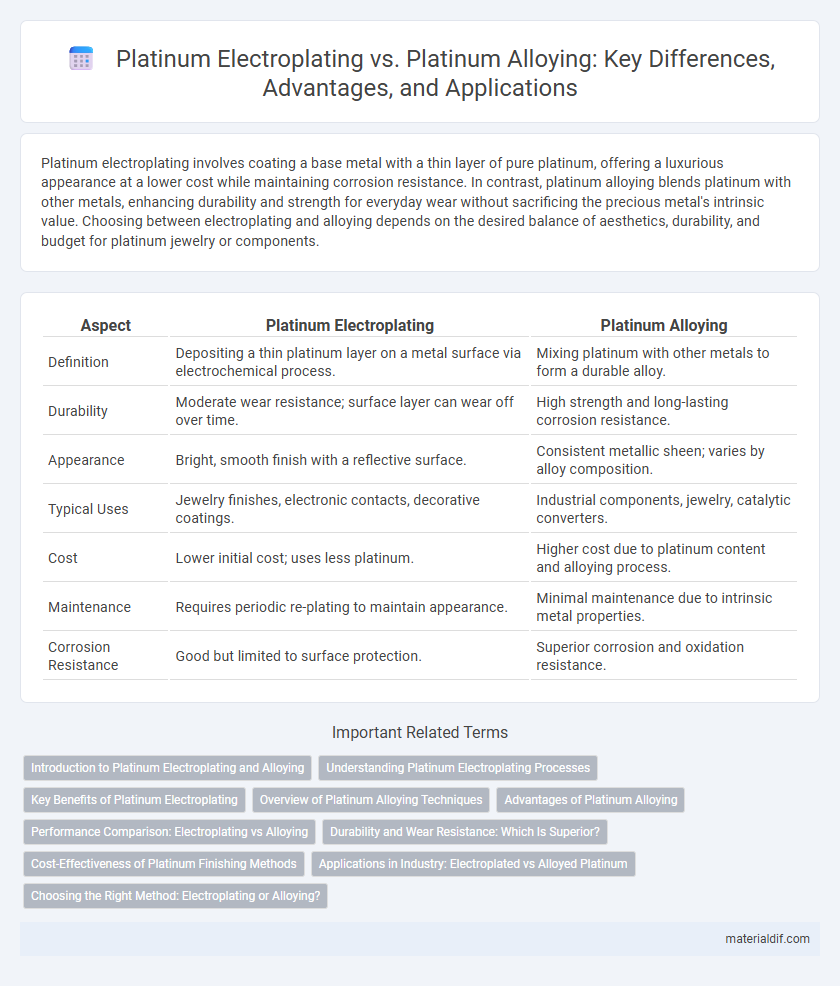
Platinum electroplating involves coating a base metal with a thin layer of pure platinum, offering a luxurious appearance at a lower cost while maintaining corrosion resistance. In contrast, platinum alloying blends platinum with other metals, enhancing durability and strength for everyday wear without sacrificing the precious metal's intrinsic value. Choosing between electroplating and alloying depends on the desired balance of aesthetics, durability, and budget for platinum jewelry or components.
Table of Comparison
| Aspect | Platinum Electroplating | Platinum Alloying |
|---|---|---|
| Definition | Depositing a thin platinum layer on a metal surface via electrochemical process. | Mixing platinum with other metals to form a durable alloy. |
| Durability | Moderate wear resistance; surface layer can wear off over time. | High strength and long-lasting corrosion resistance. |
| Appearance | Bright, smooth finish with a reflective surface. | Consistent metallic sheen; varies by alloy composition. |
| Typical Uses | Jewelry finishes, electronic contacts, decorative coatings. | Industrial components, jewelry, catalytic converters. |
| Cost | Lower initial cost; uses less platinum. | Higher cost due to platinum content and alloying process. |
| Maintenance | Requires periodic re-plating to maintain appearance. | Minimal maintenance due to intrinsic metal properties. |
| Corrosion Resistance | Good but limited to surface protection. | Superior corrosion and oxidation resistance. |
Introduction to Platinum Electroplating and Alloying
Platinum electroplating involves depositing a thin layer of pure platinum onto the surface of metals, enhancing corrosion resistance, conductivity, and aesthetic appeal without altering the base metal's core properties. Platinum alloying, in contrast, incorporates platinum into a metal matrix by melting and mixing, resulting in materials with enhanced strength, durability, and thermal stability suitable for industrial and jewelry applications. Both techniques leverage platinum's unique chemical inertness and high melting point, but electroplating focuses on surface modification while alloying changes the bulk material composition.
Understanding Platinum Electroplating Processes
Platinum electroplating involves depositing a thin layer of platinum onto the surface of another metal using an electrolytic cell, enhancing corrosion resistance and surface hardness without altering the base metal's bulk properties. This process requires precise control of parameters such as current density, electrolyte composition, temperature, and plating time to ensure uniform coating adhesion and optimal thickness. In contrast to platinum alloying, which integrates platinum atoms into a base metal's crystal lattice to modify mechanical and physical characteristics, electroplating maintains the original substrate's core structure while providing a valuable platinum surface finish.
Key Benefits of Platinum Electroplating
Platinum electroplating provides a thin, uniform layer of pure platinum that enhances corrosion resistance, improves surface hardness, and offers a superior aesthetic shine without significantly increasing the weight or cost of the base metal. This process allows for precise thickness control, ensuring optimal durability and conductivity, making it ideal for electronic components and fine jewelry. In contrast to platinum alloying, electroplating preserves the underlying material properties while delivering the premium appearance and protective benefits of platinum.
Overview of Platinum Alloying Techniques
Platinum alloying techniques involve combining pure platinum with metals such as ruthenium, iridium, or palladium to enhance hardness, durability, and corrosion resistance, crucial for industrial and jewelry applications. These alloys are produced through processes like melting and casting, powder metallurgy, and sintering, which allow precise control over composition and material properties. Compared to platinum electroplating, alloying creates a more integral and mechanically robust material structure, making it ideal for high-stress environments.
Advantages of Platinum Alloying
Platinum alloying offers enhanced durability and corrosion resistance compared to platinum electroplating, making it ideal for applications requiring long-term wear resistance, such as jewelry and industrial components. The alloyed platinum maintains its luster and structural integrity under extreme conditions, unlike electroplated surfaces that can wear off or tarnish over time. Cost-efficiency is improved through alloying, as it uses less platinum while providing similar or superior mechanical properties.
Performance Comparison: Electroplating vs Alloying
Platinum electroplating offers a highly uniform, corrosion-resistant surface ideal for enhancing conductivity and aesthetic appeal without altering the underlying substrate's mechanical properties. In contrast, platinum alloying improves overall material strength, hardness, and thermal stability by integrating platinum atoms into the metal matrix, resulting in superior wear resistance and durability. Performance comparison reveals electroplated layers excel in surface protection and electrical applications, while platinum alloys provide enhanced structural integrity for heavy-duty industrial uses.
Durability and Wear Resistance: Which Is Superior?
Platinum electroplating provides a thin, decorative layer that enhances surface hardness but may wear off over time, especially under frequent friction or abrasion. Platinum alloying integrates platinum atoms within the metal's crystal structure, significantly improving overall durability and wear resistance by increasing tensile strength and resistance to deformation. For long-term applications requiring superior wear resistance, platinum alloying generally outperforms electroplating due to its intrinsic material toughness rather than a surface-level coating.
Cost-Effectiveness of Platinum Finishing Methods
Platinum electroplating offers a cost-effective solution by applying a thin, durable platinum layer to base metals, significantly reducing the amount of precious metal used compared to solid platinum alloying. Platinum alloying involves combining platinum with other metals, resulting in higher material costs and limited flexibility in design alterations. For applications requiring luxury aesthetics with budget constraints, electroplating maximizes platinum's visual and protective properties while minimizing expenses.
Applications in Industry: Electroplated vs Alloyed Platinum
Platinum electroplating provides a thin, corrosion-resistant layer ideal for enhancing the surface properties of jewelry, electronic connectors, and medical instruments without altering the base metal's mechanical strength. In contrast, platinum alloying integrates platinum with metals like iridium or ruthenium to improve hardness and durability, making it essential for high-performance industrial catalysts, laboratory equipment, and automotive components. Both methods optimize platinum's unique properties but serve distinct applications: electroplating enhances surface aesthetics and corrosion resistance, while alloying tailors structural and thermal characteristics for demanding environments.
Choosing the Right Method: Electroplating or Alloying?
Platinum electroplating offers a thin, uniform coating ideal for enhancing surface hardness and corrosion resistance on base metals, while platinum alloying integrates platinum directly into the metal matrix, improving overall durability and melting point. Electroplating is cost-effective for decorative applications and electronics, whereas alloying suits high-performance uses in automotive and industrial sectors requiring mechanical strength. Selecting between electroplating and alloying depends on desired product attributes, budget constraints, and specific application demands.
Platinum Electroplating vs Platinum Alloying Infographic
 materialdif.com
materialdif.com