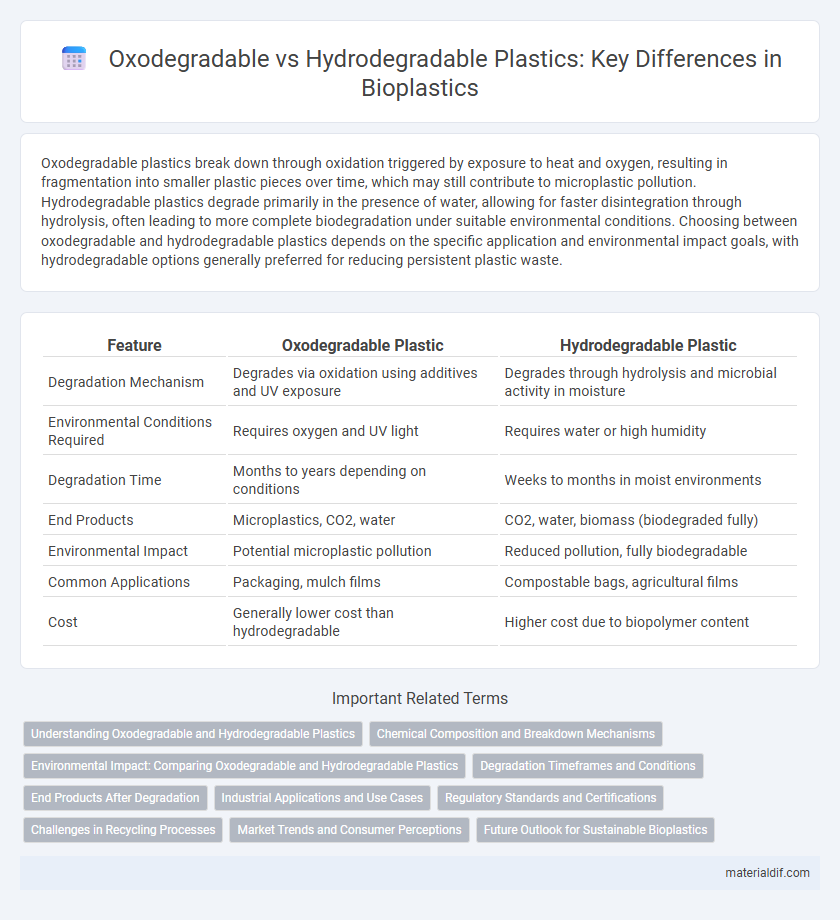
Oxodegradable plastics break down through oxidation triggered by exposure to heat and oxygen, resulting in fragmentation into smaller plastic pieces over time, which may still contribute to microplastic pollution. Hydrodegradable plastics degrade primarily in the presence of water, allowing for faster disintegration through hydrolysis, often leading to more complete biodegradation under suitable environmental conditions. Choosing between oxodegradable and hydrodegradable plastics depends on the specific application and environmental impact goals, with hydrodegradable options generally preferred for reducing persistent plastic waste.
Table of Comparison
| Feature | Oxodegradable Plastic | Hydrodegradable Plastic |
|---|---|---|
| Degradation Mechanism | Degrades via oxidation using additives and UV exposure | Degrades through hydrolysis and microbial activity in moisture |
| Environmental Conditions Required | Requires oxygen and UV light | Requires water or high humidity |
| Degradation Time | Months to years depending on conditions | Weeks to months in moist environments |
| End Products | Microplastics, CO2, water | CO2, water, biomass (biodegraded fully) |
| Environmental Impact | Potential microplastic pollution | Reduced pollution, fully biodegradable |
| Common Applications | Packaging, mulch films | Compostable bags, agricultural films |
| Cost | Generally lower cost than hydrodegradable | Higher cost due to biopolymer content |
Understanding Oxodegradable and Hydrodegradable Plastics
Oxodegradable plastics break down through oxidation triggered by heat, light, or oxygen, resulting in fragmentation into microplastics rather than complete biodegradation, posing environmental concerns. Hydrodegradable plastics degrade by absorbing moisture, leading to polymer chain cleavage and eventual decomposition by microorganisms, offering a more eco-friendly alternative. Understanding the chemical mechanisms and environmental impact distinguishes oxodegradable plastics' partial oxidation from hydrodegradable plastics' microbial assimilation.
Chemical Composition and Breakdown Mechanisms
Oxodegradable plastics contain additives like metal salts and transition metals that catalyze polymer chain scission through oxidative degradation when exposed to heat and oxygen, leading to fragmentation into microplastics. Hydrodegradable plastics incorporate hydrolyzable bonds such as ester or amide linkages that enable breakdown via hydrolysis in the presence of water, producing smaller, often biodegradable molecules. The chemical composition dictates their environmental fate: oxodegradables rely on abiotic oxidation processes, whereas hydrodegradables depend on abiotic hydrolysis followed by microbial assimilation.
Environmental Impact: Comparing Oxodegradable and Hydrodegradable Plastics
Oxodegradable plastics degrade through oxidative processes triggered by heat and UV exposure, resulting in fragmentation into microplastics that persist in the environment, potentially harming ecosystems. Hydrodegradable plastics break down via hydrolysis when exposed to moisture, leading to complete biodegradation by microorganisms and reduced environmental footprint. Studies indicate hydrodegradable plastics offer a more eco-friendly alternative by minimizing long-term pollution and facilitating natural decomposition.
Degradation Timeframes and Conditions
Oxodegradable plastics typically degrade within a few months to a couple of years through exposure to oxygen, heat, and UV light, breaking down into smaller fragments but rarely fully mineralizing, which can lead to microplastic pollution. Hydrodegradable plastics rely on moisture and microbial activity to decompose, with degradation timeframes ranging from several weeks to months, often requiring specific environmental conditions like high humidity or composting facilities to achieve complete breakdown. Understanding the distinct degradation mechanisms and timeframes is crucial for selecting environmentally appropriate bioplastics based on the intended disposal and exposure environments.
End Products After Degradation
Oxodegradable plastics break down into microplastics through oxidation, leaving residues that persist in the environment and may contribute to pollution. Hydrodegradable plastics degrade via hydrolysis, resulting in smaller polymer fragments but often do not fully mineralize, leading to incomplete decomposition. True biodegradation requires microbial activity converting these plastics into water, carbon dioxide, and biomass, which is typically limited in both oxo- and hydrodegradable materials.
Industrial Applications and Use Cases
Oxodegradable plastics are commonly used in packaging and agricultural films where gradual breakdown through oxidation is essential for reducing plastic waste. Hydrodegradable plastics find applications in medical disposables and food service items, benefiting from rapid degradation in moist environments. Industries select oxodegradable or hydrodegradable materials based on specific breakdown triggers and environmental conditions to optimize waste management.
Regulatory Standards and Certifications
Oxodegradable plastics are typically regulated under specific standards such as ASTM D6954, which evaluates their degradation through oxidation, while hydrodegradable plastics often comply with certifications like EN 13432 that assess biodegradability in composting environments. Regulatory agencies prioritize clear labeling and performance testing to ensure materials break down without leaving harmful residues, influencing market acceptance and environmental impact assessments. Compliance with these standards ensures that oxodegradable and hydrodegradable plastics meet safety, degradation timelines, and ecological criteria required for sustainable use.
Challenges in Recycling Processes
Oxodegradable plastics contain additives that promote fragmentation under UV light, complicating recycling by producing microplastics that contaminate recycling streams. Hydrodegradable plastics degrade through hydrolysis, leading to inconsistent breakdown rates that hinder sorting and recycling efficiency. Both materials pose significant challenges in existing recycling facilities due to their unpredictable degradation behavior and contamination risks.
Market Trends and Consumer Perceptions
Oxodegradable plastics are gaining market traction due to their compatibility with existing plastic manufacturing processes, appealing to industries seeking cost-effective sustainability solutions. Hydrodegradable plastics attract environmentally conscious consumers who prioritize rapid degradation in aqueous environments, particularly for single-use items. Market trends indicate a growing preference for bioplastics that balance functional performance with ecological benefits, while consumer perceptions increasingly demand transparency on degradation timelines and environmental impact.
Future Outlook for Sustainable Bioplastics
Oxodegradable plastics, which break down through oxidation triggered by heat and light, face criticism due to incomplete degradation and potential microplastic pollution, whereas hydrodegradable plastics offer enhanced biodegradability through water-based enzymatic processes but often require specific environmental conditions to degrade effectively. Future outlooks for sustainable bioplastics emphasize the development of fully compostable materials that combine both oxo- and hydrodegradability traits with bio-based feedstocks, aiming to reduce environmental impact and improve lifecycle performance. Emerging innovations focus on integrating advanced polymer chemistry and microbial technologies to create next-generation bioplastics that meet circular economy standards and regulatory frameworks worldwide.
Oxodegradable plastic vs Hydrodegradable plastic Infographic
 materialdif.com
materialdif.com