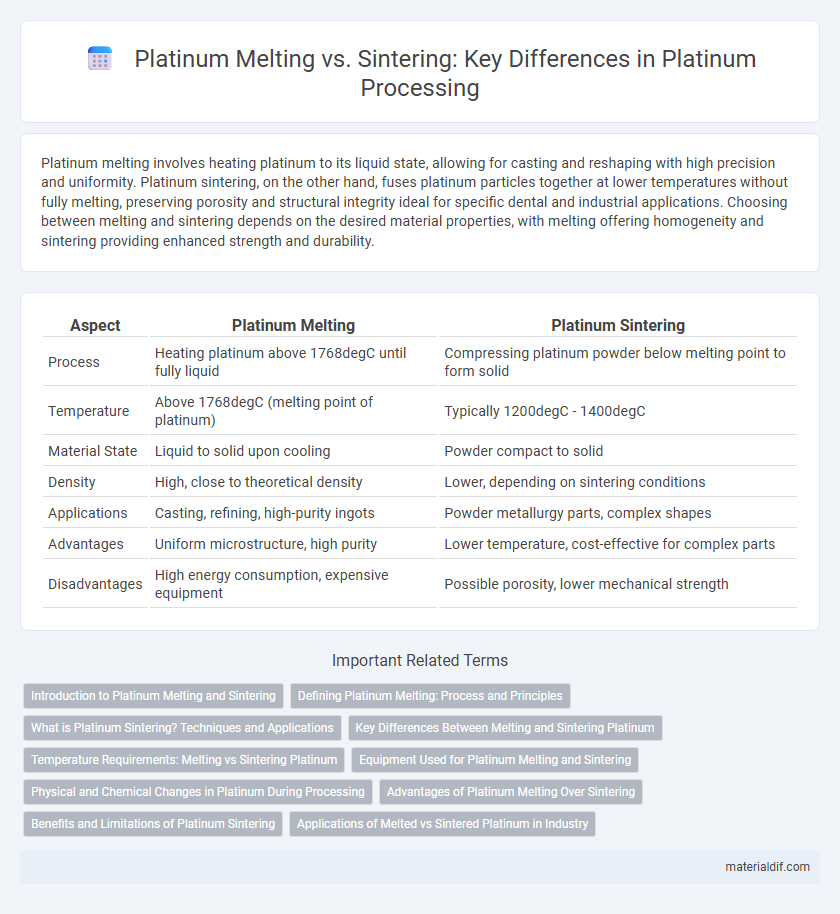
Platinum melting involves heating platinum to its liquid state, allowing for casting and reshaping with high precision and uniformity. Platinum sintering, on the other hand, fuses platinum particles together at lower temperatures without fully melting, preserving porosity and structural integrity ideal for specific dental and industrial applications. Choosing between melting and sintering depends on the desired material properties, with melting offering homogeneity and sintering providing enhanced strength and durability.
Table of Comparison
| Aspect | Platinum Melting | Platinum Sintering |
|---|---|---|
| Process | Heating platinum above 1768degC until fully liquid | Compressing platinum powder below melting point to form solid |
| Temperature | Above 1768degC (melting point of platinum) | Typically 1200degC - 1400degC |
| Material State | Liquid to solid upon cooling | Powder compact to solid |
| Density | High, close to theoretical density | Lower, depending on sintering conditions |
| Applications | Casting, refining, high-purity ingots | Powder metallurgy parts, complex shapes |
| Advantages | Uniform microstructure, high purity | Lower temperature, cost-effective for complex parts |
| Disadvantages | High energy consumption, expensive equipment | Possible porosity, lower mechanical strength |
Introduction to Platinum Melting and Sintering
Platinum melting involves heating the metal above its melting point of 1,768degC to achieve a liquid state, enabling casting and alloy formation with precise control over composition. Platinum sintering, by contrast, compacts powdered platinum below its melting temperature through heat and pressure, preserving particle integrity while promoting bonding for dense, solid structures. Understanding these fundamental processes is critical for applications requiring platinum's exceptional corrosion resistance, catalytic properties, and high-temperature stability.
Defining Platinum Melting: Process and Principles
Platinum melting involves heating the metal above its melting point of 1768degC (3214degF) to achieve a liquid state, enabling casting and alloying processes. This high-temperature phase transition requires precise control to prevent oxidation and preserve purity. The process relies on thermodynamic principles such as heat transfer, phase equilibrium, and material compatibility with refractory containers.
What is Platinum Sintering? Techniques and Applications
Platinum sintering is a manufacturing process where finely powdered platinum particles are heated below their melting point to bond and form a solid mass without fully liquefying the metal. Techniques such as spark plasma sintering and hot pressing optimize density and mechanical strength in platinum components used in catalysis, electronics, and biomedical implants. This method preserves platinum's chemical properties while enabling precise control over porosity and microstructure, essential for advanced applications.
Key Differences Between Melting and Sintering Platinum
Platinum melting involves heating the metal to its melting point of 1,768degC to achieve a fully liquid state, allowing it to be cast into precise shapes with uniform density. In contrast, platinum sintering is a solid-state process where powdered platinum is heated below its melting point, typically between 1,200degC and 1,400degC, causing particles to bond through diffusion without becoming liquid. Key differences include melting resulting in complete liquefaction and high density parts, while sintering produces porous structures with controlled shrinkage and is energy-efficient for complex shapes.
Temperature Requirements: Melting vs Sintering Platinum
Platinum melting requires extremely high temperatures around 1,768 degrees Celsius (3,214 degrees Fahrenheit) to transition from solid to liquid, making it suitable for casting and alloying processes. In contrast, platinum sintering occurs at significantly lower temperatures, typically between 1,200 and 1,400 degrees Celsius, where powdered platinum particles fuse without fully melting, preserving intricate shapes and maintaining material density. Understanding these distinct temperature requirements is crucial for selecting appropriate manufacturing techniques in platinum-based applications such as catalysis, jewelry, and electronics.
Equipment Used for Platinum Melting and Sintering
Platinum melting requires high-temperature furnaces such as induction or resistance furnaces capable of reaching temperatures above 1,700degC to achieve complete liquefaction. In contrast, platinum sintering employs specialized sintering ovens or spark plasma sintering equipment that use controlled heat and pressure to bond platinum particles without melting. Both processes necessitate precise temperature control and atmosphere regulation equipment to prevent oxidation and preserve platinum's purity.
Physical and Chemical Changes in Platinum During Processing
Platinum melting involves heating the metal above its melting point of 1,768degC, resulting in a phase transition from solid to liquid that preserves its chemical composition but alters its physical state and microstructure. Platinum sintering, in contrast, is a powder metallurgy process where platinum particles bond below the melting point through diffusion, causing densification and grain growth without melting, maintaining the metal's chemical stability. Both processes induce physical changes such as microstructural evolution and grain boundary modification while the chemical properties of platinum remain largely unchanged due to its high chemical inertness.
Advantages of Platinum Melting Over Sintering
Platinum melting offers superior homogeneity and density compared to sintering, resulting in materials with enhanced mechanical strength and corrosion resistance. This process eliminates porosity inherent in sintered parts, leading to improved thermal and electrical conductivity essential for high-performance applications. Melting also enables precise control over alloy composition, ensuring consistent quality and performance in industrial and jewelry uses.
Benefits and Limitations of Platinum Sintering
Platinum sintering offers enhanced structural integrity and uniform porosity, making it ideal for catalytic applications and high-precision components where controlled microstructure is essential. Unlike melting, sintering avoids high-temperature liquefaction, reducing energy consumption and minimizing grain growth, which preserves mechanical strength and surface area. However, limitations include potential incomplete densification and residual porosity, which can affect conductivity and corrosion resistance compared to fully melted and cast platinum.
Applications of Melted vs Sintered Platinum in Industry
Melted platinum, known for its superior density and structural integrity, is widely used in high-performance catalytic converters and precision electronics, where durability and conductivity are critical. Sintered platinum, produced through powder metallurgy, offers enhanced porosity and surface area, making it ideal for fuel cell electrodes and chemical catalyst supports. The choice between melted and sintered platinum depends on application-specific demands for mechanical strength versus surface reactivity in industrial processes.
Platinum Melting vs Platinum Sintering Infographic
 materialdif.com
materialdif.com