Cobalt offers a sleek, durable finish that is ideal for modern pet accessories, while Platinum is prized for its superior strength and hypoallergenic properties, making it perfect for pets with sensitive skin. Platinum's resistance to tarnish and corrosion ensures long-lasting shine and durability compared to Cobalt. Choosing between Cobalt and Platinum depends on the desired balance of aesthetic appeal and long-term wear resistance in pet products.
Table of Comparison
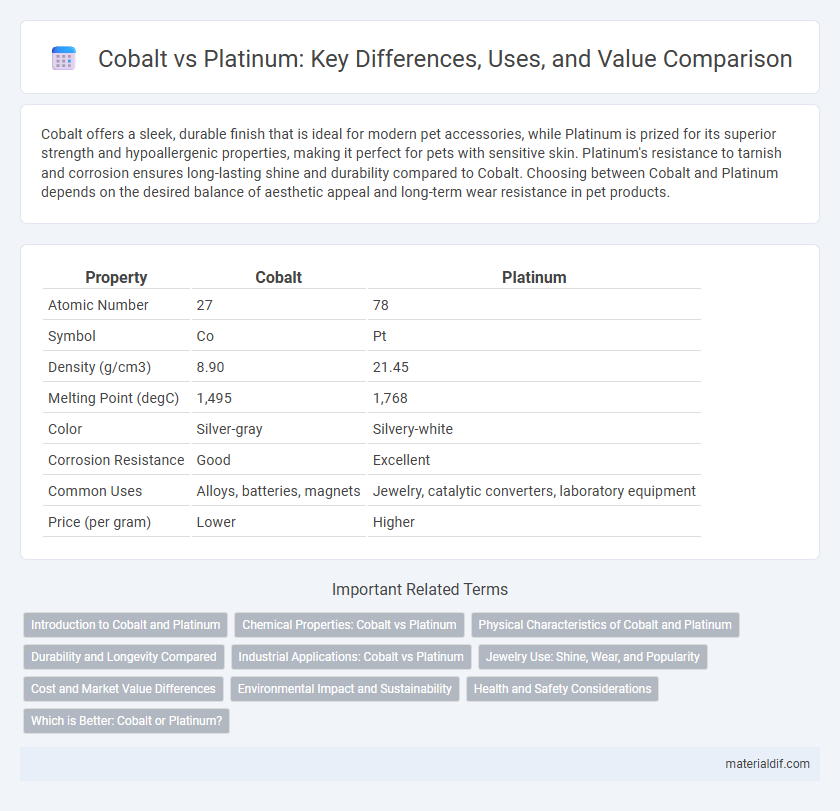
| Property | Cobalt | Platinum |
|---|---|---|
| Atomic Number | 27 | 78 |
| Symbol | Co | Pt |
| Density (g/cm3) | 8.90 | 21.45 |
| Melting Point (degC) | 1,495 | 1,768 |
| Color | Silver-gray | Silvery-white |
| Corrosion Resistance | Good | Excellent |
| Common Uses | Alloys, batteries, magnets | Jewelry, catalytic converters, laboratory equipment |
| Price (per gram) | Lower | Higher |
Introduction to Cobalt and Platinum
Cobalt is a transition metal known for its magnetic properties and significant use in batteries, alloys, and catalysts, while platinum is a dense, malleable noble metal prized for its corrosion resistance and catalytic efficiency in industrial applications. Both metals belong to the d-block elements but exhibit distinct characteristics; cobalt is primarily blue-gray and ferromagnetic, whereas platinum is silvery-white with high ductility and exceptional chemical stability. Industrial demand for cobalt centers on its role in electronics and renewable energy, while platinum's value is driven by automotive catalytic converters, jewelry, and chemical processes.
Chemical Properties: Cobalt vs Platinum
Cobalt and platinum both exhibit unique chemical properties essential to industrial applications; cobalt has an atomic number of 27 and typically forms +2 and +3 oxidation states, making it highly reactive in forming magnetic alloys and batteries. Platinum, with an atomic number of 78, is a noble metal renowned for its resistance to corrosion and oxidation, commonly exhibiting +2 and +4 oxidation states in catalytic processes. The distinct electron configurations and reactivity levels of cobalt and platinum define their roles in chemical reactions and material science.
Physical Characteristics of Cobalt and Platinum
Cobalt is a hard, lustrous, silver-gray metal with a melting point of 1495degC and a density of 8.9 g/cm3, known for its magnetic properties at room temperature. Platinum is a dense, malleable, and highly corrosion-resistant metal with a melting point of 1768degC and a density of 21.45 g/cm3, exhibiting excellent conductivity and a distinctive silvery-white appearance. Both metals are transition elements, but platinum's superior density and higher melting point make it ideal for high-temperature and catalytic applications, while cobalt's magnetic characteristics are leveraged in electronics and alloys.
Durability and Longevity Compared
Cobalt exhibits exceptional hardness and resistance to wear, making it highly durable for industrial applications, but it is more prone to oxidation compared to platinum. Platinum offers superior corrosion resistance and maintains its luster over time, contributing to greater longevity in jewelry and medical devices. While cobalt's toughness suits heavy-duty use, platinum's balance of strength and chemical stability ensures long-term performance and minimal maintenance.
Industrial Applications: Cobalt vs Platinum
Cobalt is extensively utilized in battery production, aerospace components, and hard metals due to its magnetic properties and high-temperature resistance. Platinum excels in catalytic converters, chemical processing, and fuel cells because of its exceptional catalytic activity and corrosion resistance. While cobalt is favored for energy storage and magnetic applications, platinum remains a critical material in emission control and chemical synthesis industries.
Jewelry Use: Shine, Wear, and Popularity
Platinum is highly valued in jewelry for its exceptional shine and durability, maintaining its luster longer than cobalt, which tends to dull over time. Unlike cobalt, platinum is hypoallergenic, making it a preferred choice for sensitive skin and high-end pieces. Its heavier weight and natural white sheen contribute to its sustained popularity in luxury jewelry markets worldwide.
Cost and Market Value Differences
Cobalt generally costs less per ounce than platinum, driven by its high demand in battery manufacturing and relatively abundant supply. Platinum commands a higher market value due to its rarity, extensive industrial uses in automotive catalytic converters, and jewelry. Market volatility affects both metals, but platinum's price fluctuations are more influenced by geopolitical factors and automotive industry trends.
Environmental Impact and Sustainability
Cobalt mining poses significant environmental challenges due to toxic waste and habitat destruction, while platinum extraction typically results in lower ecological damage because of more efficient recycling methods and less toxic byproducts. Platinum is highly sustainable in catalytic converters and hydrogen fuel cells, enabling cleaner energy solutions with longer-lasting material life cycles compared to cobalt-based batteries. The environmental footprint of platinum is further reduced by advances in eco-friendly refining techniques and increased industry focus on circular supply chains.
Health and Safety Considerations
Cobalt exposure presents significant health risks including skin dermatitis, respiratory issues, and potential carcinogenic effects, necessitating strict workplace safety measures. Platinum, while generally less toxic, can cause allergic reactions and respiratory problems, especially in its salt forms, requiring controlled handling and proper ventilation. Both metals demand rigorous monitoring to prevent occupational hazards, but cobalt's higher toxicity levels make health and safety considerations more critical in industrial environments.
Which is Better: Cobalt or Platinum?
Platinum offers superior corrosion resistance and exceptional durability compared to cobalt, making it ideal for high-end jewelry and industrial applications. Cobalt, however, boasts higher strength and affordability, often used in battery production and medical implants. Choosing between cobalt and platinum depends on whether cost-efficiency or long-lasting luxury and chemical stability are prioritized.
Cobalt vs Platinum Infographic
 materialdif.com
materialdif.com