Electroforming creates a thick, durable layer of silver by depositing metal onto a mold, resulting in robust and intricate silver pet accessories. Electroplating applies a thin silver coating to a base metal, offering a shiny finish but less durability compared to electroforming. For long-lasting and high-quality silver pet items, electroforming is the preferred technique due to its strength and detail retention.
Table of Comparison
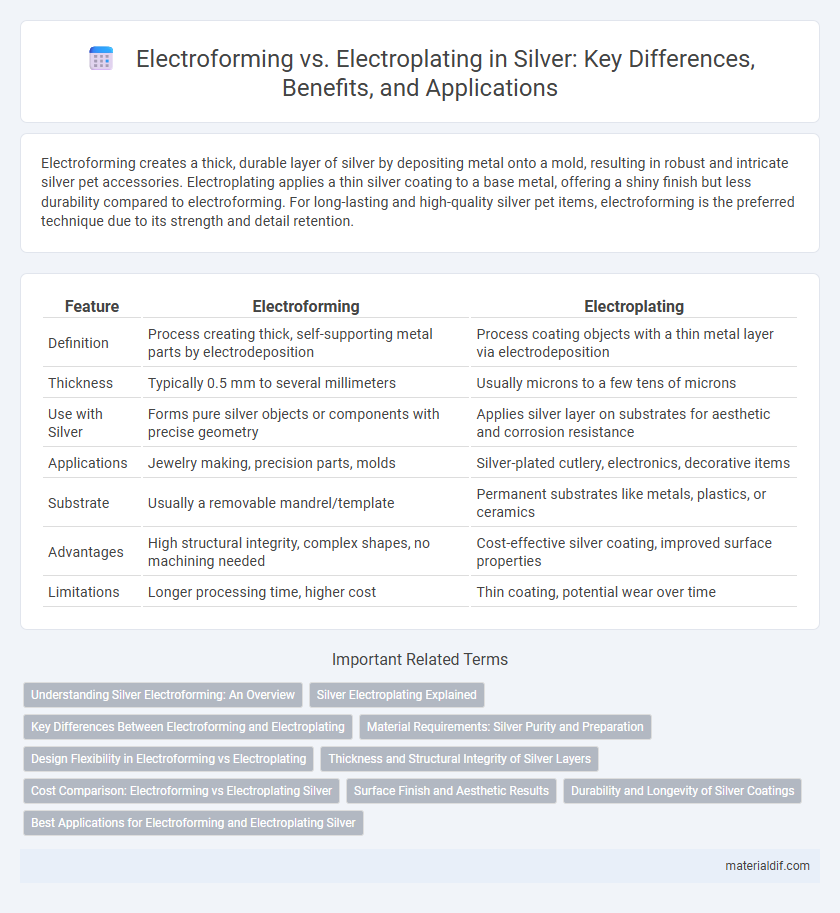
| Feature | Electroforming | Electroplating |
|---|---|---|
| Definition | Process creating thick, self-supporting metal parts by electrodeposition | Process coating objects with a thin metal layer via electrodeposition |
| Thickness | Typically 0.5 mm to several millimeters | Usually microns to a few tens of microns |
| Use with Silver | Forms pure silver objects or components with precise geometry | Applies silver layer on substrates for aesthetic and corrosion resistance |
| Applications | Jewelry making, precision parts, molds | Silver-plated cutlery, electronics, decorative items |
| Substrate | Usually a removable mandrel/template | Permanent substrates like metals, plastics, or ceramics |
| Advantages | High structural integrity, complex shapes, no machining needed | Cost-effective silver coating, improved surface properties |
| Limitations | Longer processing time, higher cost | Thin coating, potential wear over time |
Understanding Silver Electroforming: An Overview
Silver electroforming involves depositing a thick layer of pure silver onto a conductive mold through electrolytic deposition, creating detailed and durable metal structures ideal for jewelry and decorative arts. Unlike electroplating, which applies a thin silver coating primarily for aesthetic enhancement and corrosion resistance, electroforming builds substantial silver components with precise control over thickness and complex shapes. This technique leverages silver's excellent conductivity and malleability, enabling intricate designs that maintain structural integrity and high-quality finishes.
Silver Electroplating Explained
Silver electroplating involves depositing a thin layer of silver onto a conductive surface using an electrolytic bath containing silver ions, enhancing the substrate with corrosion resistance and improved conductivity. Unlike electroforming, which builds a thick metallic layer or entire object, electroplating primarily focuses on coating existing items with a uniform silver film for decorative or functional purposes. Key parameters include current density, bath temperature, and silver concentration, which directly influence the plating's quality, brightness, and adhesion.
Key Differences Between Electroforming and Electroplating
Electroforming builds thick, structural metal layers by depositing silver onto a mandrel, enabling the creation of standalone silver objects, whereas electroplating applies a thin silver coating onto existing metal surfaces for decorative or protective purposes. The electroforming process allows precise control over thickness and shape, forming complex silver components not achievable with electroplating. Electroplating typically enhances aesthetics and corrosion resistance, while electroforming produces durable silver parts with substantial mechanical strength.
Material Requirements: Silver Purity and Preparation
Electroforming requires high-purity silver, typically 99.9% or above, to ensure optimal conductivity and structural integrity during the deposition process. The silver surface must be meticulously cleaned and often chemically polished to remove oxides and contaminants, enhancing adhesion and uniform layer buildup. In contrast, electroplating can tolerate slightly lower purity levels around 99.5%, with less rigorous surface preparation, as the plated layer is thinner and primarily decorative.
Design Flexibility in Electroforming vs Electroplating
Electroforming offers superior design flexibility compared to electroplating by enabling the creation of intricate and detailed shapes through metal deposition on a model, which can be removed after forming. Silver electroforming allows precise control over thickness and texture, resulting in complex, lightweight components that are difficult to achieve with electroplating. In contrast, silver electroplating applies a thin, uniform layer over existing surfaces, limiting the ability to form unique shapes or significant structural modifications.
Thickness and Structural Integrity of Silver Layers
Electroforming produces thicker, mechanically stronger silver layers compared to electroplating, often exceeding 100 microns in thickness, which enhances durability and structural integrity. Electroplating typically deposits thinner silver coatings ranging from 0.1 to 10 microns, suitable for surface decoration but less robust under mechanical stress. The superior thickness of electroformed silver enables complex, self-supporting structures ideal for precision applications in electronics and jewelry.
Cost Comparison: Electroforming vs Electroplating Silver
Electroforming silver generally incurs higher initial setup costs due to specialized equipment and longer processing times, while electroplating silver offers a more cost-efficient alternative for thin, uniform coatings with faster production cycles. Material consumption in electroplating is lower, reducing silver waste and making it more economical for large-scale applications. Maintenance and operational expenses tend to be higher in electroforming, impacting overall cost-effectiveness depending on project complexity and volume.
Surface Finish and Aesthetic Results
Electroforming silver produces a thicker, more uniform surface finish with intricate detail retention, ideal for high-precision art pieces and jewelry. Electroplating yields a thinner, smoother silver layer primarily used for enhancing surface aesthetics and corrosion resistance. The aesthetic results of electroforming tend to showcase depth and texture, while electroplating offers a polished, reflective silver coating.
Durability and Longevity of Silver Coatings
Electroforming produces thicker, more durable silver coatings by depositing metal layer-by-layer, resulting in enhanced resistance to wear and corrosion compared to electroplating. Silver electroforms exhibit superior longevity due to their robust structural integrity and reduced risk of peeling or flaking under mechanical stress. Electroplating, while faster and cost-effective, typically yields thinner silver layers that are more susceptible to tarnishing and abrasion over time.
Best Applications for Electroforming and Electroplating Silver
Electroforming silver is ideal for creating intricate, lightweight jewelry and detailed decorative objects due to its ability to build thick, uniform layers with precision. Electroplating silver is best suited for enhancing the surface of everyday items like cutlery, electronics, and coins, providing corrosion resistance and a bright, durable finish. Both methods maximize silver's conductivity and aesthetic appeal, but electroforming excels in structural depth while electroplating offers cost-effective surface enhancement.
Electroforming vs Electroplating Infographic
 materialdif.com
materialdif.com