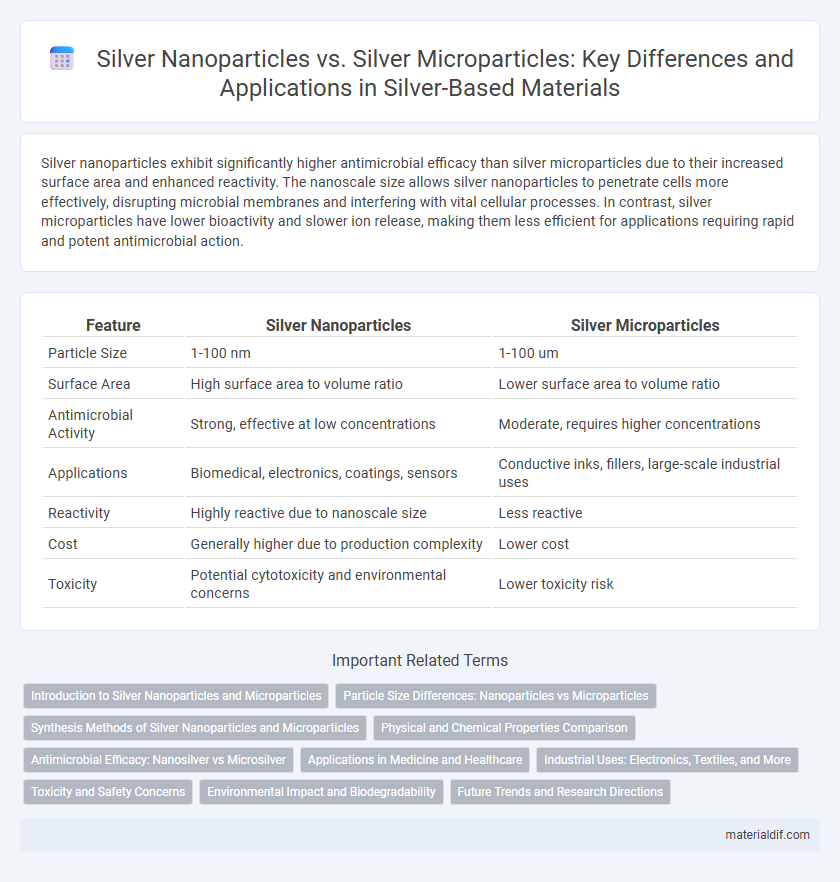
Silver nanoparticles exhibit significantly higher antimicrobial efficacy than silver microparticles due to their increased surface area and enhanced reactivity. The nanoscale size allows silver nanoparticles to penetrate cells more effectively, disrupting microbial membranes and interfering with vital cellular processes. In contrast, silver microparticles have lower bioactivity and slower ion release, making them less efficient for applications requiring rapid and potent antimicrobial action.
Table of Comparison
| Feature | Silver Nanoparticles | Silver Microparticles |
|---|---|---|
| Particle Size | 1-100 nm | 1-100 um |
| Surface Area | High surface area to volume ratio | Lower surface area to volume ratio |
| Antimicrobial Activity | Strong, effective at low concentrations | Moderate, requires higher concentrations |
| Applications | Biomedical, electronics, coatings, sensors | Conductive inks, fillers, large-scale industrial uses |
| Reactivity | Highly reactive due to nanoscale size | Less reactive |
| Cost | Generally higher due to production complexity | Lower cost |
| Toxicity | Potential cytotoxicity and environmental concerns | Lower toxicity risk |
Introduction to Silver Nanoparticles and Microparticles
Silver nanoparticles exhibit unique physicochemical properties due to their nanoscale size, including increased surface area and enhanced antimicrobial effectiveness, distinguishing them from silver microparticles, which have larger dimensions and lower reactivity. These nanoscale particles typically range between 1 to 100 nanometers, enabling superior interaction with microbial membranes and catalytic surfaces compared to microparticles that measure in the micrometer scale. Silver microparticles, while still valuable for applications like coatings and electronics, lack the pronounced surface-to-volume ratio and surface energy that give silver nanoparticles their distinctive functional advantages in medical and industrial uses.
Particle Size Differences: Nanoparticles vs Microparticles
Silver nanoparticles typically measure between 1 to 100 nanometers, exhibiting unique physicochemical properties due to their high surface area-to-volume ratio. In contrast, silver microparticles range from 1 to 100 micrometers, resulting in significantly lower surface reactivity and different optical, antimicrobial, and catalytic behaviors. The nanoscale size of silver nanoparticles enhances their penetration and interaction with microbial cells, making them more effective in applications such as medical coatings and sensors than their larger microparticle counterparts.
Synthesis Methods of Silver Nanoparticles and Microparticles
Silver nanoparticles are commonly synthesized using chemical reduction, green synthesis, and physical methods such as laser ablation, enabling precise control over particle size and shape. In contrast, silver microparticles are typically produced via mechanical milling, spray pyrolysis, or chemical precipitation techniques that yield larger, less uniform particles. The choice of synthesis method critically impacts the silver particles' surface area, reactivity, and applications in fields like medicine, electronics, and catalysis.
Physical and Chemical Properties Comparison
Silver nanoparticles exhibit a significantly larger surface area-to-volume ratio compared to silver microparticles, enhancing their chemical reactivity and catalytic efficiency. Physically, silver nanoparticles range from 1 to 100 nanometers in size, enabling distinctive optical properties such as localized surface plasmon resonance, whereas silver microparticles, typically over 1 micrometer, lack these nanoscale phenomena. Chemically, silver nanoparticles demonstrate superior antimicrobial activity due to their increased ion release and cellular interaction potential, contrasting with the comparatively inert behavior of silver microparticles.
Antimicrobial Efficacy: Nanosilver vs Microsilver
Silver nanoparticles exhibit significantly higher antimicrobial efficacy compared to silver microparticles due to their increased surface area to volume ratio, which facilitates greater interaction with microbial cell membranes. Nanosilver particles release silver ions more efficiently, leading to enhanced disruption of bacterial metabolic processes and cell structures. This targeted ion release and superior surface reactivity make silver nanoparticles particularly effective against a broad spectrum of pathogens, surpassing the antimicrobial capabilities of microsilver particles.
Applications in Medicine and Healthcare
Silver nanoparticles exhibit superior antimicrobial properties compared to silver microparticles, making them highly effective in wound dressings, coatings for medical devices, and antibacterial agents in healthcare settings. Their nanoscale size enhances surface area and reactivity, enabling targeted drug delivery, improved diagnostic imaging, and reduced cytotoxicity. Silver microparticles, while less reactive, remain valuable in traditional antiseptic applications and sustained-release formulations due to their stability and controlled ion release.
Industrial Uses: Electronics, Textiles, and More
Silver nanoparticles exhibit superior electrical conductivity and antimicrobial properties compared to silver microparticles, making them ideal for advanced electronics, such as flexible circuits and conductive inks. In textiles, silver nanoparticles are widely used for their enhanced antibacterial effects and durability, providing long-lasting odor control and hygiene in fabrics. Industrial applications extend to coatings and catalysts where nanoparticles outperform microparticles due to their higher surface area and reactivity, optimizing performance across sectors like sensors and water treatment.
Toxicity and Safety Concerns
Silver nanoparticles exhibit higher toxicity compared to silver microparticles due to their increased surface area and enhanced cellular uptake, leading to greater reactive oxygen species generation and potential DNA damage. Safety concerns highlight that nanoparticles can penetrate biological barriers more efficiently, raising risks of bioaccumulation and adverse effects on human health and the environment. Regulatory agencies emphasize strict exposure limits and thorough risk assessments to mitigate the toxicological impact of silver nanoparticles.
Environmental Impact and Biodegradability
Silver nanoparticles exhibit higher reactivity and toxicity in aquatic environments compared to silver microparticles due to their smaller size and increased surface area, leading to greater potential bioaccumulation and ecological disruption. The biodegradability of silver nanoparticles is limited, resulting in prolonged environmental persistence and challenges in natural degradation processes, whereas silver microparticles tend to settle faster and have reduced mobility, minimizing widespread contamination. Studies indicate that the environmental impact of silver nanoparticles demands cautious regulation to prevent adverse effects on microbial communities and aquatic organisms.
Future Trends and Research Directions
Silver nanoparticles exhibit superior antimicrobial properties and higher surface area-to-volume ratios compared to silver microparticles, driving ongoing research into their biomedical and environmental applications. Future trends emphasize enhancing synthesis methods for uniform size distribution, improving biocompatibility, and exploring hybrid nanomaterials to optimize efficacy in wound healing, drug delivery, and water purification. Emerging studies focus on addressing toxicity concerns and environmental impact to ensure safe and sustainable use of silver nanoparticles in advanced technological solutions.
Silver Nanoparticles vs Silver Microparticles Infographic
 materialdif.com
materialdif.com