Crystalline quartz features a highly ordered atomic structure, resulting in distinct geometric shapes and excellent piezoelectric properties, while amorphous quartz lacks this regular arrangement, appearing more glass-like and exhibiting different optical and mechanical characteristics. The crystalline form is commonly used in electronics and timekeeping devices due to its stability and precision, whereas amorphous quartz, often found in fused silica, is prized for its clarity and resistance to thermal shock. Understanding these differences is essential for selecting the appropriate form of quartz in industrial and technological applications.
Table of Comparison
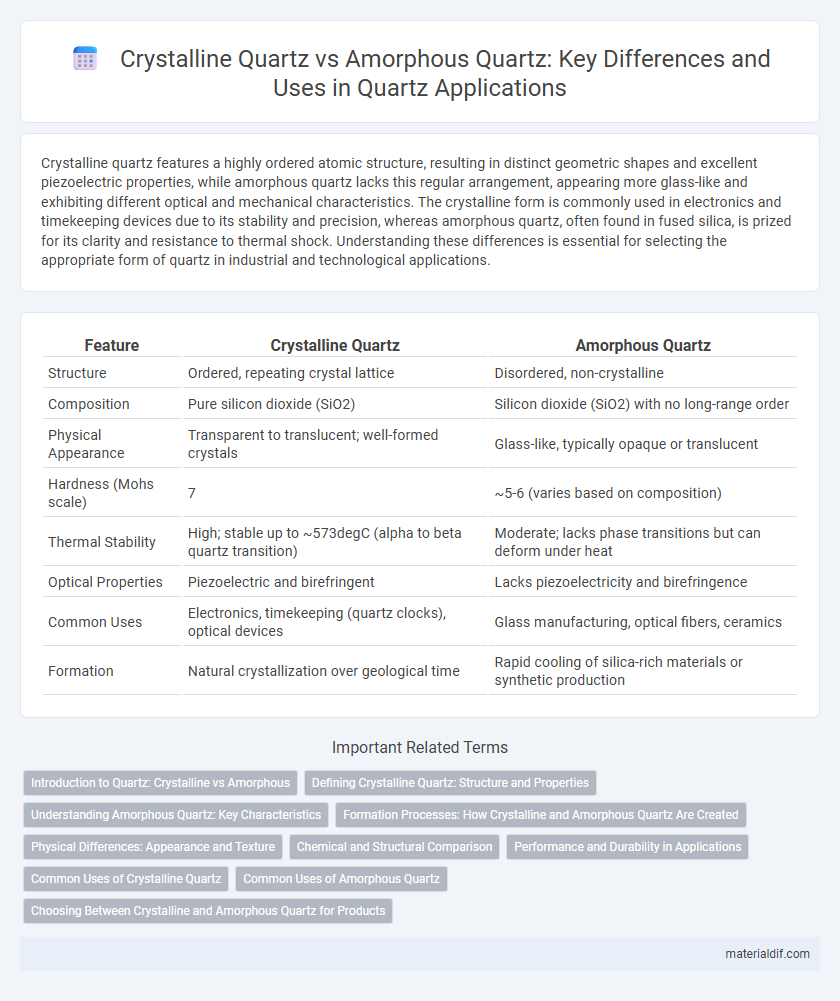
| Feature | Crystalline Quartz | Amorphous Quartz |
|---|---|---|
| Structure | Ordered, repeating crystal lattice | Disordered, non-crystalline |
| Composition | Pure silicon dioxide (SiO2) | Silicon dioxide (SiO2) with no long-range order |
| Physical Appearance | Transparent to translucent; well-formed crystals | Glass-like, typically opaque or translucent |
| Hardness (Mohs scale) | 7 | ~5-6 (varies based on composition) |
| Thermal Stability | High; stable up to ~573degC (alpha to beta quartz transition) | Moderate; lacks phase transitions but can deform under heat |
| Optical Properties | Piezoelectric and birefringent | Lacks piezoelectricity and birefringence |
| Common Uses | Electronics, timekeeping (quartz clocks), optical devices | Glass manufacturing, optical fibers, ceramics |
| Formation | Natural crystallization over geological time | Rapid cooling of silica-rich materials or synthetic production |
Introduction to Quartz: Crystalline vs Amorphous
Quartz exists primarily in two forms: crystalline and amorphous, each distinguished by its atomic structure. Crystalline quartz features a highly ordered lattice arrangement, resulting in defined geometric shapes and consistent physical properties, while amorphous quartz lacks this periodic atomic order, leading to irregular structures such as those found in fused quartz or natural silica glass. These structural differences influence their optical clarity, thermal stability, and industrial applications, with crystalline quartz favored in piezoelectric devices and amorphous quartz prized for its chemical purity and resistance to thermal shock.
Defining Crystalline Quartz: Structure and Properties
Crystalline quartz is characterized by a well-ordered atomic structure, where silicon and oxygen atoms form a continuous three-dimensional framework of SiO4 tetrahedra arranged in a hexagonal lattice. This precise atomic arrangement results in distinct physical properties, including high hardness (7 on the Mohs scale), piezoelectricity, and optical clarity, making it essential in electronics and timekeeping devices. Unlike amorphous quartz, which lacks long-range order, crystalline quartz exhibits defined cleavage planes and consistent crystal growth patterns that determine its mechanical and optical behavior.
Understanding Amorphous Quartz: Key Characteristics
Amorphous quartz, unlike its crystalline counterpart, lacks a well-defined crystal structure, resulting in a non-periodic atomic arrangement. This form of quartz is commonly found as natural silica glass or in synthetic variants such as fused quartz, exhibiting high thermal stability and resistance to chemical corrosion. Its unique structural properties make amorphous quartz essential in optical fibers, semiconductor manufacturing, and high-precision laboratory equipment.
Formation Processes: How Crystalline and Amorphous Quartz Are Created
Crystalline quartz forms through the slow cooling and solidification of silica-rich solutions, allowing atoms to arrange in a highly ordered, repeating lattice structure. Amorphous quartz, such as opal, results from rapid cooling or deposition, preventing the formation of a regular crystal lattice and creating a disordered atomic arrangement. These distinct formation processes influence the physical properties and applications of each type, with crystalline quartz exhibiting higher structural stability and hardness.
Physical Differences: Appearance and Texture
Crystalline quartz exhibits a well-defined geometric structure with smooth, faceted surfaces and a glassy luster, while amorphous quartz lacks a regular crystal lattice, resulting in a more dull, uneven texture. The transparent to translucent nature of crystalline quartz contrasts sharply with the often opaque or milky appearance of amorphous quartz. Physical hardness is consistent in both forms, but the clarity and texture differences are key indicators for identification and use in various industrial applications.
Chemical and Structural Comparison
Crystalline quartz features a highly ordered atomic structure where silicon and oxygen atoms form a continuous three-dimensional network of SiO4 tetrahedra arranged in a hexagonal lattice. In contrast, amorphous quartz lacks this long-range order, presenting a disordered network of SiO2 without a defined crystal lattice. Chemically, both share the same silicon dioxide composition, but crystalline quartz exhibits well-defined physical properties due to its structured bonding, while amorphous quartz displays isotropic properties and variable density resulting from its random atomic arrangement.
Performance and Durability in Applications
Crystalline quartz exhibits superior performance and durability in applications due to its ordered atomic structure, which provides enhanced mechanical strength, thermal stability, and piezoelectric properties. Amorphous quartz, lacking long-range order, generally has lower hardness and thermal resistance, making it less suitable for high-stress or high-temperature environments. Therefore, crystalline quartz is preferred in precision instruments, electronics, and industrial applications demanding robust and reliable material performance.
Common Uses of Crystalline Quartz
Crystalline quartz, known for its well-ordered atomic structure, is widely used in electronics, particularly in oscillators and frequency control devices like watches and radios due to its piezoelectric properties. Its durability and resistance to weathering make it a popular material in optical lenses, glass manufacturing, and precision instruments. Unlike amorphous quartz, which lacks a consistent structure, crystalline quartz offers superior mechanical and electrical stability essential for high-tech and industrial applications.
Common Uses of Amorphous Quartz
Amorphous quartz, primarily found in forms such as silica glass and opal, is widely used in the electronics industry for manufacturing optical fibers, semiconductor components, and laboratory glassware due to its high purity and excellent thermal stability. Its non-crystalline structure enables superior transmission of ultraviolet light, making it essential in UV-grade lenses and coatings. Amorphous quartz also plays a crucial role in producing durable, heat-resistant glass for industrial applications and consumer products like cookware and glassware.
Choosing Between Crystalline and Amorphous Quartz for Products
Crystalline quartz features a well-ordered atomic structure that provides superior mechanical strength, piezoelectric properties, and thermal stability, making it ideal for precision instruments, electronics, and optical devices. Amorphous quartz, lacking a defined crystalline lattice, offers greater flexibility in shape and is more resistant to thermal shock, which is advantageous for glassware, lenses, and fiber optics. Selecting between crystalline and amorphous quartz depends on the product's performance requirements, as crystalline quartz excels in high-precision, stable environments, while amorphous quartz suits applications needing durability and form versatility.
Crystalline Quartz vs Amorphous Quartz Infographic
 materialdif.com
materialdif.com