Gold electroplating creates a thick, durable layer of gold by using an electric current to deposit gold ions onto a metal surface, offering excellent corrosion resistance and a classic shiny finish. Gold ion plating, also known as Physical Vapor Deposition (PVD), applies a thinner, more uniform coating through a vaporized gold process, resulting in enhanced scratch resistance and longer-lasting color retention. Choosing between the two depends on the desired durability, thickness, and application, with electroplating favored for traditional aesthetics and ion plating preferred for modern, high-performance uses.
Table of Comparison
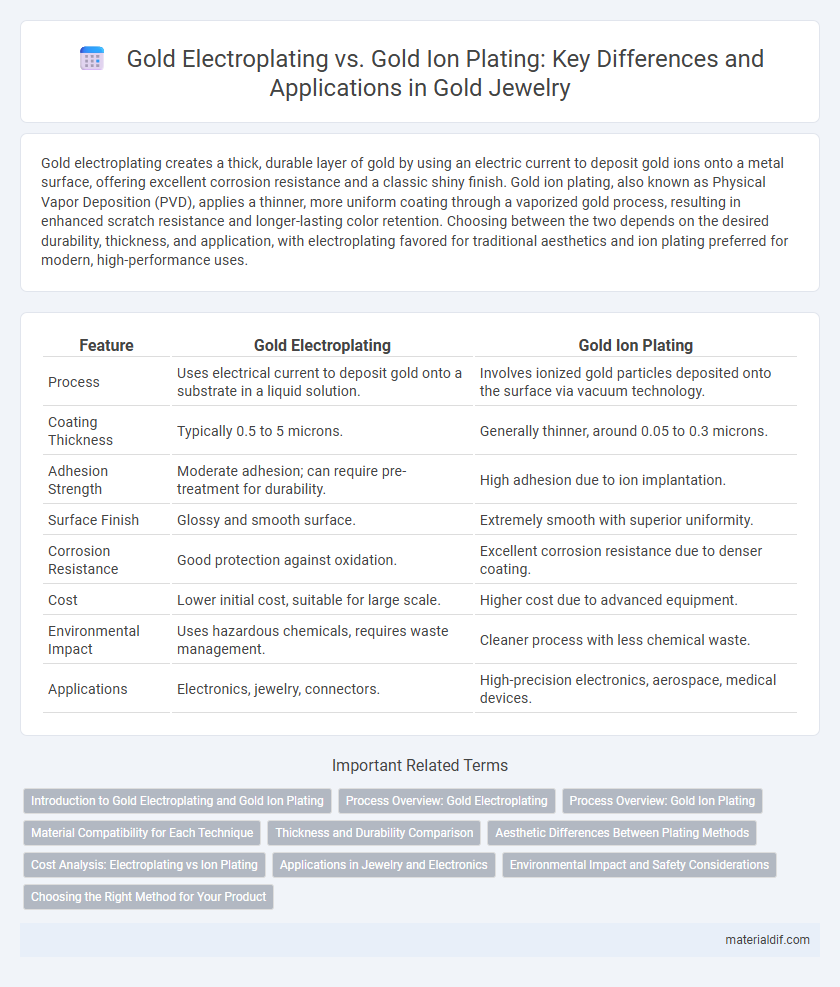
| Feature | Gold Electroplating | Gold Ion Plating |
|---|---|---|
| Process | Uses electrical current to deposit gold onto a substrate in a liquid solution. | Involves ionized gold particles deposited onto the surface via vacuum technology. |
| Coating Thickness | Typically 0.5 to 5 microns. | Generally thinner, around 0.05 to 0.3 microns. |
| Adhesion Strength | Moderate adhesion; can require pre-treatment for durability. | High adhesion due to ion implantation. |
| Surface Finish | Glossy and smooth surface. | Extremely smooth with superior uniformity. |
| Corrosion Resistance | Good protection against oxidation. | Excellent corrosion resistance due to denser coating. |
| Cost | Lower initial cost, suitable for large scale. | Higher cost due to advanced equipment. |
| Environmental Impact | Uses hazardous chemicals, requires waste management. | Cleaner process with less chemical waste. |
| Applications | Electronics, jewelry, connectors. | High-precision electronics, aerospace, medical devices. |
Introduction to Gold Electroplating and Gold Ion Plating
Gold electroplating involves depositing a thin layer of gold onto a substrate through an electrochemical process, enhancing corrosion resistance, conductivity, and aesthetic appeal. Gold ion plating, or gold ion beam deposition, uses ionized gold particles accelerated onto the surface, offering superior adhesion and precise thickness control in high-tech applications. Both techniques improve surface properties, but gold ion plating is preferred for advanced electronics due to its uniform coating and enhanced durability.
Process Overview: Gold Electroplating
Gold electroplating involves immersing a substrate into a solution containing gold ions, where an electric current reduces the gold ions onto the surface, creating a thin and uniform gold layer. This process offers precise control over thickness, excellent adhesion, and enhanced corrosion resistance. Unlike gold ion plating, electroplating relies on an external power source to deposit gold, making it ideal for jewelry, electronics, and decorative applications requiring durability and conductivity.
Process Overview: Gold Ion Plating
Gold ion plating utilizes a physical vapor deposition (PVD) process where gold ions are vaporized in a vacuum chamber and deposited onto a substrate, resulting in a thin, uniform coating with enhanced adhesion and durability. This technique offers superior corrosion resistance and precise thickness control compared to traditional electroplating, which relies on electrochemical reduction to deposit gold from a liquid solution. The PVD method employed in gold ion plating produces environmentally friendly coatings with improved hardness and wear resistance, making it ideal for high-performance applications in electronics and jewelry.
Material Compatibility for Each Technique
Gold electroplating requires conductive substrates such as copper, nickel, or silver to ensure effective deposition, while non-conductive materials are typically unsuitable without pre-treatment. Gold ion plating, also known as physical vapor deposition (PVD), offers broader material compatibility, enabling coating on both conductive and non-conductive surfaces like plastics, ceramics, and glass. This versatility makes gold ion plating preferable for delicate or non-metallic components that cannot withstand the harsh chemical baths used in electroplating.
Thickness and Durability Comparison
Gold electroplating typically achieves thicknesses ranging from 0.5 to 5 microns, offering substantial durability suitable for heavy wear applications and corrosion resistance. Gold ion plating, or PVD (Physical Vapor Deposition), deposits ultra-thin layers generally between 0.05 and 0.5 microns, providing excellent surface hardness and improved wear resistance despite thinner coatings. The thicker electroplated layers excel in mechanical durability, while ion plating offers enhanced adhesion and corrosion protection with minimal material usage.
Aesthetic Differences Between Plating Methods
Gold electroplating offers a smooth, bright finish with a classic yellow hue that enhances jewelry and decorative items, while gold ion plating delivers a more uniform, durable coating with richer color saturation and improved resistance to wear. The ion plating method produces a harder surface and sharper detail retention, making it preferable for high-precision applications where aesthetic longevity is critical. In contrast, electroplating creates a softer, more traditional gold appearance but may require more frequent maintenance to preserve its shine.
Cost Analysis: Electroplating vs Ion Plating
Gold electroplating is generally more cost-effective due to lower equipment and operational expenses, making it suitable for large-scale production with simpler coatings. Gold ion plating, while offering superior durability and finer control over coating thickness, requires higher initial investment and maintenance costs, impacting overall affordability. The choice between electroplating and ion plating hinges on balancing budget constraints against desired coating quality and product performance.
Applications in Jewelry and Electronics
Gold electroplating offers a thick, durable coating ideal for high-wear jewelry and reliable electronic contacts, providing excellent corrosion resistance and enhanced conductivity. Gold ion plating, utilizing advanced PVD technology, creates a thinner, more uniform layer with superior hardness and scratch resistance, making it perfect for delicate jewelry pieces and precision electronic components. Both methods optimize aesthetic appeal and functionality, but choice depends on balancing coating thickness, durability, and application-specific demands in jewelry and electronics.
Environmental Impact and Safety Considerations
Gold electroplating involves hazardous chemicals such as cyanide and heavy metals, posing significant environmental disposal challenges and worker safety risks due to toxic fumes and waste. Gold ion plating, also known as ion beam assisted deposition, uses physical vapor deposition without toxic chemicals, resulting in a safer and more environmentally friendly process with minimal hazardous waste. Choosing gold ion plating reduces ecological footprint and enhances workplace safety by eliminating cyanide and limiting chemical exposure.
Choosing the Right Method for Your Product
Gold electroplating involves depositing a thin layer of gold onto a substrate using an electric current, ideal for achieving a uniform, durable coating on complex shapes. Gold ion plating, a more advanced technique, uses ionized gold particles in a vacuum chamber for a highly precise, ultra-thin coating with superior adhesion and corrosion resistance. Selecting the right method depends on your product's requirements for thickness, durability, surface finish, and cost efficiency.
Gold electroplating vs Gold ion plating Infographic
 materialdif.com
materialdif.com