Gold nanoparticles exhibit unique optical, chemical, and physical properties distinct from bulk gold due to their nanoscale size and high surface area-to-volume ratio. These properties enable enhanced catalytic activity, improved bioavailability, and tunable plasmonic effects, making gold nanoparticles highly valuable in biomedical and technological applications. In contrast, bulk gold remains chemically inert, with stable conducting properties suitable for traditional uses in jewelry and electronics.
Table of Comparison
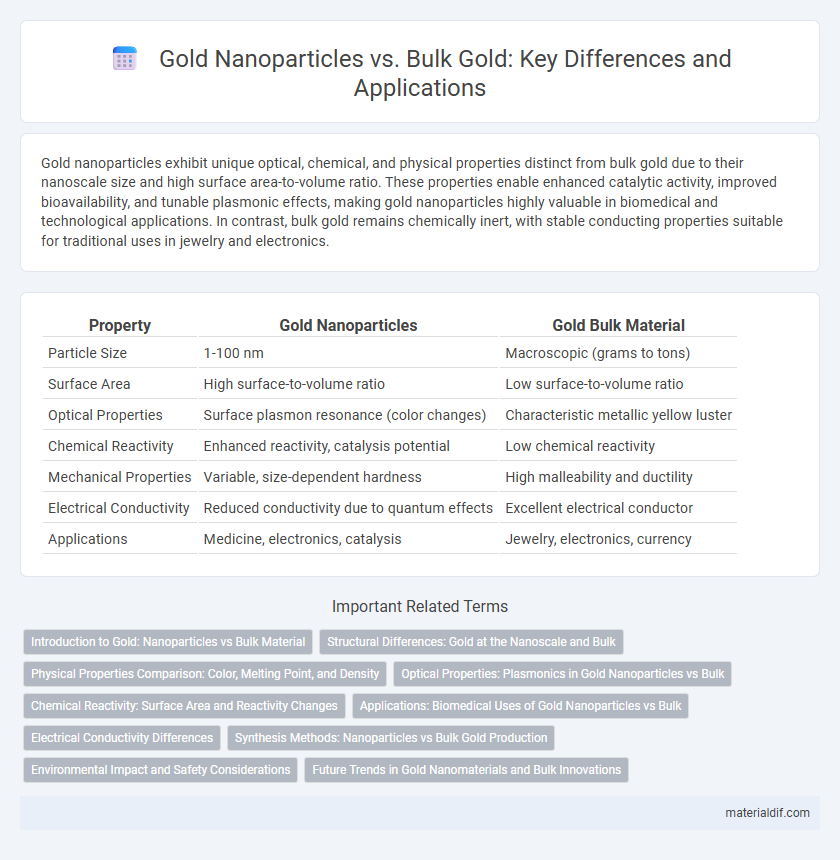
| Property | Gold Nanoparticles | Gold Bulk Material |
|---|---|---|
| Particle Size | 1-100 nm | Macroscopic (grams to tons) |
| Surface Area | High surface-to-volume ratio | Low surface-to-volume ratio |
| Optical Properties | Surface plasmon resonance (color changes) | Characteristic metallic yellow luster |
| Chemical Reactivity | Enhanced reactivity, catalysis potential | Low chemical reactivity |
| Mechanical Properties | Variable, size-dependent hardness | High malleability and ductility |
| Electrical Conductivity | Reduced conductivity due to quantum effects | Excellent electrical conductor |
| Applications | Medicine, electronics, catalysis | Jewelry, electronics, currency |
Introduction to Gold: Nanoparticles vs Bulk Material
Gold nanoparticles exhibit unique optical, electronic, and chemical properties distinct from bulk gold due to their high surface area to volume ratio and quantum size effects. Unlike bulk gold, which is primarily valued for its conductivity, malleability, and inertness, gold nanoparticles display size-dependent plasmonic resonance, allowing applications in biomedical imaging, drug delivery, and catalysis. The distinct physicochemical characteristics of gold nanoparticles enable innovative technological advancements not achievable with bulk gold material.
Structural Differences: Gold at the Nanoscale and Bulk
Gold nanoparticles exhibit a high surface-to-volume ratio, resulting in unique structural properties such as increased surface atom mobility and altered crystal facets compared to bulk gold. At the nanoscale, gold atoms arrange in configurations that can include pentagonal and icosahedral symmetries, distinct from the face-centered cubic (FCC) lattice structure predominant in bulk gold. These structural differences significantly influence the nanoparticles' optical, catalytic, and mechanical behaviors, distinguishing them from their bulk material counterparts.
Physical Properties Comparison: Color, Melting Point, and Density
Gold nanoparticles exhibit distinct physical properties compared to bulk gold, notably in color, melting point, and density. While bulk gold displays its characteristic metallic yellow hue, gold nanoparticles often appear red or purple due to surface plasmon resonance effects. The melting point of gold nanoparticles significantly decreases as particle size reduces, dropping from the bulk gold melting point of 1,064degC to below 300degC for particles under 5 nm, while density variations are minimal but can slightly decrease due to surface atom relaxation.
Optical Properties: Plasmonics in Gold Nanoparticles vs Bulk
Gold nanoparticles exhibit localized surface plasmon resonance (LSPR), resulting in strong optical absorption and scattering at specific wavelengths, unlike bulk gold, which shows broad plasmonic behavior due to free electron oscillations. The tunable plasmonic properties of gold nanoparticles depend on their size, shape, and dielectric environment, enabling applications in sensing, imaging, and photothermal therapy. Bulk gold's optical response is less sensitive to environmental changes, limiting its plasmonic versatility compared to nanoscale counterparts.
Chemical Reactivity: Surface Area and Reactivity Changes
Gold nanoparticles exhibit significantly enhanced chemical reactivity compared to bulk gold material due to their substantially higher surface area-to-volume ratio, which exposes more active sites for catalytic reactions. This increased surface area enables gold nanoparticles to interact more effectively with reactants, facilitating processes such as oxidation and reduction at lower temperatures and with greater efficiency. The altered electronic properties at the nanoscale further contribute to the unique reactivity profile of gold nanoparticles, distinguishing them from their bulk counterparts in various chemical applications.
Applications: Biomedical Uses of Gold Nanoparticles vs Bulk
Gold nanoparticles exhibit unique optical and chemical properties that enable targeted drug delivery, enhanced imaging contrast, and photothermal therapy in biomedical applications, outperforming bulk gold materials. Their nanoscale size allows for cellular and molecular level interaction, facilitating early disease diagnosis and precise treatment with minimal side effects. Bulk gold, while biocompatible and corrosion-resistant, lacks the surface reactivity and versatility required for advanced therapeutic and diagnostic techniques.
Electrical Conductivity Differences
Gold nanoparticles exhibit lower electrical conductivity compared to bulk gold material due to quantum size effects and increased electron scattering at nanoparticle surfaces. Bulk gold's conductivity benefits from its continuous crystal lattice, allowing free electron flow with minimal resistance. Surface plasmon resonance in gold nanoparticles also alters electron mobility, impacting their electrical conduction properties relative to bulk counterparts.
Synthesis Methods: Nanoparticles vs Bulk Gold Production
Gold nanoparticles are commonly synthesized using bottom-up methods such as chemical reduction, where gold ions are reduced to form nanoscale particles with controlled size and shape, often employing stabilizing agents like citrate or thiols. In contrast, bulk gold production typically involves mining and refining processes including cyanidation and electrorefining to obtain large, pure gold masses. The synthesis of gold nanoparticles allows precise manipulation of optical, electronic, and catalytic properties, whereas bulk gold production emphasizes purity and macroscopic form for industrial and jewelry applications.
Environmental Impact and Safety Considerations
Gold nanoparticles exhibit distinct environmental and safety profiles compared to bulk gold, as their nanoscale size increases reactivity and potential toxicity to aquatic organisms. Unlike bulk gold, which is chemically inert and poses minimal ecological risks, gold nanoparticles can penetrate biological membranes, leading to bioaccumulation and oxidative stress in ecosystems. Regulatory frameworks emphasize cautious handling and disposal of gold nanoparticles to mitigate environmental contamination and human exposure.
Future Trends in Gold Nanomaterials and Bulk Innovations
Gold nanoparticles exhibit unique optical, electronic, and catalytic properties distinct from bulk gold, driving advancements in biomedical applications, electronics, and environmental sensing. Future trends in gold nanomaterials include enhanced targeted drug delivery systems, improved photothermal therapies, and scalable green synthesis methods. Concurrently, innovations in bulk gold focus on sustainable extraction techniques, alloy development for improved durability, and integration into flexible electronics.
Gold Nanoparticles vs Gold Bulk Material Infographic
 materialdif.com
materialdif.com