Graphite exhibits a crystalline structure with layers of carbon atoms arranged in a hexagonal lattice, providing excellent electrical conductivity and high thermal stability. Amorphous carbon lacks this ordered structure, resulting in lower conductivity but a higher surface area beneficial for applications like adsorption and catalysis. The distinct properties between graphite and amorphous carbon influence their usage across industries such as electronics, energy storage, and environmental technologies.
Table of Comparison
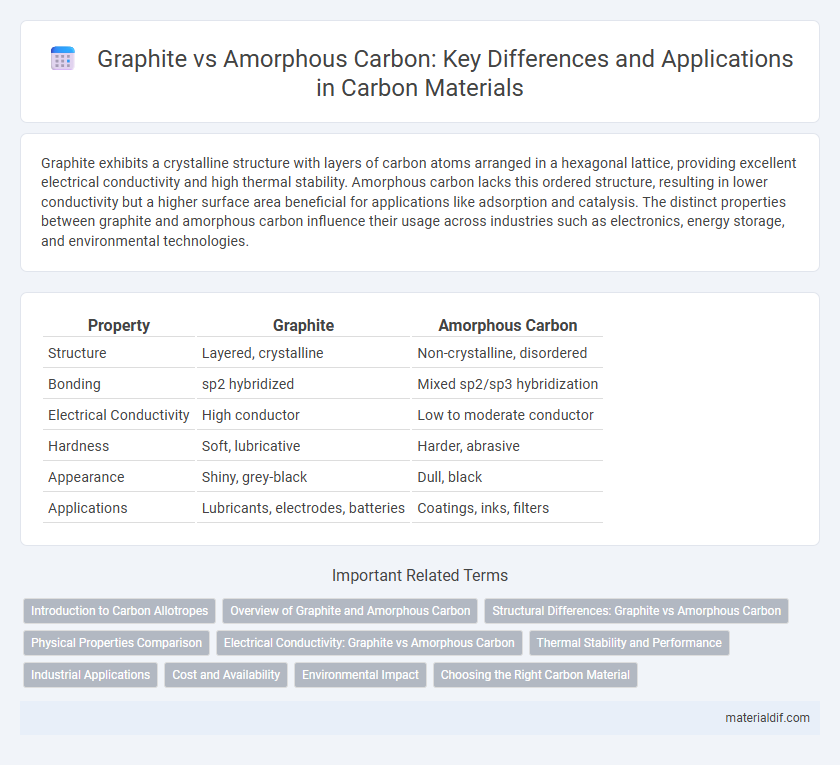
| Property | Graphite | Amorphous Carbon |
|---|---|---|
| Structure | Layered, crystalline | Non-crystalline, disordered |
| Bonding | sp2 hybridized | Mixed sp2/sp3 hybridization |
| Electrical Conductivity | High conductor | Low to moderate conductor |
| Hardness | Soft, lubricative | Harder, abrasive |
| Appearance | Shiny, grey-black | Dull, black |
| Applications | Lubricants, electrodes, batteries | Coatings, inks, filters |
Introduction to Carbon Allotropes
Graphite and amorphous carbon are two primary allotropes of carbon, each exhibiting distinct structural and physical properties. Graphite features a crystalline arrangement of hexagonal layers with delocalized electrons, enabling high electrical conductivity and lubricating properties. Amorphous carbon lacks a long-range ordered structure, resulting in varied properties used in applications such as carbon black and activated carbon for filtration and reinforcement.
Overview of Graphite and Amorphous Carbon
Graphite is a crystalline form of carbon characterized by its layered hexagonal lattice structure, providing excellent electrical conductivity and lubricating properties. Amorphous carbon lacks a defined crystalline structure, resulting in a more disordered arrangement of carbon atoms that affects its hardness and electrical behavior. These structural differences fundamentally influence their applications, with graphite commonly used in electrodes and lubricants, while amorphous carbon finds use in coatings and composite materials.
Structural Differences: Graphite vs Amorphous Carbon
Graphite features a crystalline structure composed of hexagonally arranged carbon atoms forming layers with strong covalent bonds within the planes and weak van der Waals forces between them. Amorphous carbon lacks long-range order, presenting a disordered arrangement of carbon atoms without a defined lattice. The structural difference results in graphite's high electrical conductivity and lubricative properties, whereas amorphous carbon exhibits lower conductivity and enhanced hardness due to its random bonding.
Physical Properties Comparison
Graphite features a layered, crystalline structure with high electrical conductivity, excellent thermal stability, and a lubricating effect due to weak van der Waals forces between layers. Amorphous carbon lacks long-range order, resulting in lower electrical conductivity, higher hardness, and increased chemical reactivity compared to graphite. The density of graphite ranges around 2.2 g/cm3, while amorphous carbon typically exhibits a denser, less uniform structure with variable densities between 1.8 and 2.1 g/cm3.
Electrical Conductivity: Graphite vs Amorphous Carbon
Graphite exhibits high electrical conductivity due to its well-ordered layered structure with delocalized electrons enabling efficient electron mobility. In contrast, amorphous carbon lacks this crystalline arrangement, resulting in lower electrical conductivity because electron movement is hindered by structural disorder and defects. This difference makes graphite a preferred material for electrical applications, whereas amorphous carbon is often used where insulation or lower conductivity is needed.
Thermal Stability and Performance
Graphite exhibits superior thermal stability due to its highly ordered crystalline structure, allowing it to withstand temperatures above 3000degC without significant degradation. In contrast, amorphous carbon lacks long-range order, resulting in lower thermal stability and performance, typically deteriorating at temperatures below 1500degC. The enhanced thermal conductivity and stability of graphite make it ideal for high-temperature applications compared to the more reactive and less stable amorphous carbon.
Industrial Applications
Graphite's crystalline structure provides excellent electrical conductivity and thermal resistance, making it ideal for electrodes in electric arc furnaces and batteries. Amorphous carbon, with its disordered atomic arrangement, offers superior hardness and wear resistance, suitable for coatings, lubricants, and abrasive materials. Industrial applications favor graphite for high-temperature processes and electrochemical uses, while amorphous carbon excels in protective films and mechanical durability.
Cost and Availability
Graphite is generally more expensive than amorphous carbon due to its crystalline structure and higher purity required in industrial applications. Amorphous carbon, widely available as a byproduct of incomplete combustion, offers a cost-effective alternative for uses like pigments and batteries. Both materials have distinct market demands, with graphite's limited natural deposits impacting its price and amorphous carbon benefiting from abundant, low-cost sources.
Environmental Impact
Graphite exhibits a more structured molecular arrangement, which typically results in lower environmental degradation during extraction and processing compared to amorphous carbon. Amorphous carbon sources, often derived from less controlled processes like coal combustion, tend to generate higher pollutant emissions and greater ecological disturbance. Sustainable graphite mining practices and synthetic graphite production can significantly reduce carbon emissions and habitat disruption relative to amorphous carbon extraction.
Choosing the Right Carbon Material
Graphite offers high electrical conductivity and thermal stability, making it ideal for applications in batteries, electrodes, and lubricants, whereas amorphous carbon provides superior hardness and chemical resistance suited for coatings and composite materials. Selecting the right carbon material depends on specific performance requirements such as conductivity, durability, and surface properties. Understanding the material's microstructure and intended application environment ensures optimal functionality and longevity.
Graphite vs Amorphous Carbon Infographic
 materialdif.com
materialdif.com